Lysophosphatidic acid receptor 1 antagonist (EPGN2154) causes regression of NASH in preclinical NASH models
- PMID: 37994050
- PMCID: PMC10666985
- DOI: 10.1097/HC9.0000000000000323
Lysophosphatidic acid receptor 1 antagonist (EPGN2154) causes regression of NASH in preclinical NASH models
Abstract
Background: NASH causes a tremendous health care burden in the United States. A glucagon-like peptide-1 agonist, semaglutide (Sema), treatment resulted in hepatic steatosis reduction in clinical trials of NASH. Lysophosphatidic acid receptor 1 antagonists are known to have antifibrotic effects in several organs. We tested Sema and a novel lysophosphatidic acid receptor 1 antagonist, EPGN2154, individually and in combination to evaluate their efficacy for NASH remission in preclinical models.
Methods: In the present study, we used (1) C57Bl6/J wild-type mice fed on a high-fat, high-carbohydrate (HFHC) diet for 16 weeks and (2) leptin-deficient mice (ob/ob) fed on an Amylin liver NASH diet for 16 weeks. After 16 weeks, the mice were randomly distributed in equal numbers in (1) no-drug, (2) EPGN2154, (3) Sema, and (4) EPGN2154+Sema treatment groups for 8 additional weeks at a dosage of 10 mg/kg body weight for EPGN2154 (oral gavage, 5 days a week) and 6.17 μg/kg body weight of Sema (subcutaneous injection every alternate day, 3 days a week).
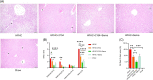
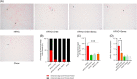
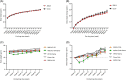
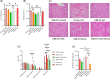
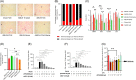
Results: In the wild-type-high-fat, high-carbohydrate model, we observed the most body weight loss in the EPGN2154+Sema combination group compared to the other treatment groups. All groups led to a significant reduction in alanine transaminase levels when compared to high-fat, high-carbohydrate-fed wild type. However, no significant difference in alanine transaminase levels was observed among the treatment groups. In the ob/ob mice study, Sema did not cause body weight loss. Moreover, the EPGN2154 and the combination groups had a lower NAFLD Activity Score and incidence of advanced-stage hepatic fibrosis than the Sema group.
Conclusions: EPGN2154 demonstrated a hepato-protective effect independent of body weight loss in preclinical NASH models.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Association for the Study of Liver Diseases.
Conflict of interest statement
Graham Beaton: cofounder, Epigen Biosciences Inc.; Satheesh B. Ravula: cofounder, Epigen Biosciences Inc.; Suk Joong Lee: employee, Epigen Biosciences Inc.; Kevin B. Bacon: ex-employee, Epigen Biosciences Inc.; Celia P. Jenkinson: ex-employee, Epigen Biosciences Inc. and employee/owns stock, Travere Therapeutics; Fabio C. Tucci: cofounder, Epigen Biosciences Inc. and owns stock, Bruin Biosciences; Rohit Kohli: consultant, Epigen Biosciences Inc. The remaining authors have no conflicts to report.
Figures



References
-
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. - PubMed
-
- Staels B, Rubenstrunk A, Noel B, Rigou G, Delataille P, Millatt LJ, et al. . Hepatoprotective effects of the dual peroxisome proliferator-activated receptor alpha/delta agonist, GFT505, in rodent models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology [Internet]. 2013;58:1941–52. - PubMed
-
- Cable EE, Finn PD, Stebbins JW, Hou J, Ito BR, van Poelje PD, et al. . Reduction of hepatic steatosis in rats and mice after treatment with a liver-targeted thyroid hormone receptor agonist. Hepatology. 2009;49:407–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

