Innate immune cellular therapeutics in transplantation
- PMID: 37994308
- PMCID: PMC10664839
- DOI: 10.3389/frtra.2023.1067512
Innate immune cellular therapeutics in transplantation
Abstract
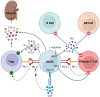
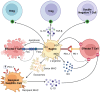
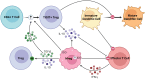
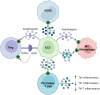
Successful organ transplantation provides an opportunity to extend the lives of patients with end-stage organ failure. Selectively suppressing the donor-specific alloimmune response, however, remains challenging without the continuous use of non-specific immunosuppressive medications, which have multiple adverse effects including elevated risks of infection, chronic kidney injury, cardiovascular disease, and cancer. Efforts to promote allograft tolerance have focused on manipulating the adaptive immune response, but long-term allograft survival rates remain disappointing. In recent years, the innate immune system has become an attractive therapeutic target for the prevention and treatment of transplant organ rejection. Indeed, contemporary studies demonstrate that innate immune cells participate in both the initial alloimmune response and chronic allograft rejection and undergo non-permanent functional reprogramming in a phenomenon termed "trained immunity." Several types of innate immune cells are currently under investigation as potential therapeutics in transplantation, including myeloid-derived suppressor cells, dendritic cells, regulatory macrophages, natural killer cells, and innate lymphoid cells. In this review, we discuss the features and functions of these cell types, with a focus on their role in the alloimmune response. We examine their potential application as therapeutics to prevent or treat allograft rejection, as well as challenges in their clinical translation and future directions for investigation.
Keywords: MDSCs (myeloid-derived suppressor cells); cellular therapeutics; human monocyte-derived suppressor cells; innate lymphoid cells (ILCs); regulatory dendritic cells; regulatory macrophages; transplantation.
Conflict of interest statement
Conflict of interest The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources

