Idiosyncratic bone responses to blood flow restriction exercise: new insights and future directions
- PMID: 37994414
- PMCID: PMC11212818
- DOI: 10.1152/japplphysiol.00723.2022
Idiosyncratic bone responses to blood flow restriction exercise: new insights and future directions
Abstract
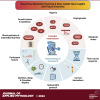
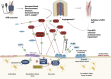
Applying blood flow restriction (BFR) during low-load exercise induces beneficial adaptations of the myotendinous and neuromuscular systems. Despite the low mechanical tension, BFR exercise facilitates a localized hypoxic environment and increase in metabolic stress, widely regarded as the primary stimulus for tissue adaptations. First evidence indicates that low-load BFR exercise is effective in promoting an osteogenic response in bone, although this has previously been postulated to adapt primarily during high-impact weight-bearing exercise. Besides studies investigating the acute response of bone biomarkers following BFR exercise, first long-term trials demonstrate beneficial adaptations in bone in both healthy and clinical populations. Despite the increasing number of studies, the physiological mechanisms are largely unknown. Moreover, heterogeneity in methodological approaches such as biomarkers of bone metabolism measured, participant and study characteristics, and time course of measurement renders it difficult to formulate accurate conclusions. Furthermore, incongruity in the methods of BFR application (e.g., cuff pressure) limits the comparability of datasets and thus hinders generalizability of study findings. Appropriate use of biomarkers, effective BFR application, and befitting study design have the potential to progress knowledge on the acute and chronic response of bone to BFR exercise and contribute toward the development of a novel strategy to protect or enhance bone health. Therefore, the purpose of the present synthesis review is to 1) evaluate current mechanistic evidence; 2) discuss and offer explanations for similar and contrasting data findings; and 3) create a methodological framework for future mechanistic and applied research.
Keywords: blood flow restriction; bone remodeling; exercise; hypoxia.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


References
-
- Daly RM, Rosengren BE, Alwis G, Ahlborg HG, Sernbo I, Karlsson MK. Gender specific age-related changes in bone density, muscle strength and functional performance in the elderly: a-10 year prospective population-based study. BMC Geriatr 13: 71, 2013. doi: 10.1186/1471-2318-13-71. - DOI - PMC - PubMed
-
- Wolff J. The Law of Bone Remodelling. Heidelberg: Springer, 1986.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

