Frequent Premature Atrial Contractions Lead to Adverse Atrial Remodeling and Atrial Fibrillation in a Swine Model
- PMID: 37994608
- PMCID: PMC10872765
- DOI: 10.1161/CIRCULATIONAHA.123.065874
Frequent Premature Atrial Contractions Lead to Adverse Atrial Remodeling and Atrial Fibrillation in a Swine Model
Abstract
Background: Frequent premature atrial complexes (PACs) are associated with future incident atrial fibrillation (AF), but whether PACs contribute to development of AF through adverse atrial remodeling has not been studied. This study aimed to explore the effect of frequent PACs from different sites on atrial remodeling in a swine model.
Methods: Forty swine underwent baseline electrophysiologic studies and echocardiography followed by pacemaker implantations and paced PACs (50% burden) at 250-ms coupling intervals for 16 weeks in 4 groups: (1) lateral left atrium (LA) PACs by the coronary sinus (Lat-PAC; n=10), (2) interatrial septal PACs (Sep-PAC; n=10), (3) regular LA pacing at 130 beats/min (Reg-130; n=10), and (4) controls without PACs (n=10). At the final study, repeat studies were performed, followed by tissue histology and molecular analyses focusing on fibrotic pathways.
Results: Lat-PACs were associated with a longer P-wave duration (93.0±9.0 versus 74.2±8.2 and 58.8±7.6 ms; P<0.001) and greater echocardiographic mechanical dyssynchrony (57.5±11.6 versus 35.7±13.0 and 24.4±11.1 ms; P<0.001) compared with Sep-PACs and controls, respectively. After 16 weeks, Lat-PACs led to slower LA conduction velocity (1.1±0.2 versus 1.3±0.2 [Sep-PAC] versus 1.3±0.1 [Reg-130] versus 1.5±0.2 [controls] m/s; P<0.001) without significant change in atrial ERP. The Lat-PAC group had a significantly increased percentage of LA fibrosis and upregulated levels of extracellular matrix proteins (lysyl oxidase and collagen 1 and 8), as well as TGF-β1 (transforming growth factor-β1) signaling proteins (latent and monomer TGF-β1 and phosphorylation/total ratio of SMAD2/3; P<0.05). The Lat-PAC group had the longest inducible AF duration (terminal to baseline: 131 [interquartile range 30, 192] seconds versus 16 [6, 26] seconds [Sep-PAC] versus 22 [11, 64] seconds [Reg-130] versus -1 [-16, 7] seconds [controls]; P<0.001).
Conclusions: In this swine model, frequent PACs resulted in adverse atrial structural remodeling with a heightened propensity to AF. PACs originating from the lateral LA produced greater atrial remodeling and longer induced AF duration than the septal-origin PACs. These data provide evidence that frequent PACs can cause adverse atrial remodeling as well as AF, and that the location of ectopic PACs may be clinically meaningful.
Keywords: atrial fibrillation; atrial premature contractions; atrial remodeling.
Conflict of interest statement
Figures



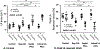
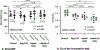
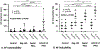
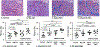
References
-
- Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998. Sep 3;339(10):659–66. doi: 10.1056/NEJM199809033391003. - DOI - PubMed
-
- Dewland TA, Vittinghoff E, Mandyam MC, Heckbert SR, Siscovick DS, Stein PK, Psaty BM, Sotoodehnia N, Gottdiener JS, Marcus GM. Atrial ectopy as a predictor of incident atrial fibrillation: a cohort study. Ann Intern Med. 2013. Dec 3;159(11):721–8. doi: 10.7326/0003-4819-159-11-201312030-00004. - DOI - PMC - PubMed
-
- Murakoshi N, Xu D, Sairenchi T, Igarashi M, Irie F, Tomizawa T, Tada H, Sekiguchi Y, Yamagishi K, Iso H, Yamaguchi I, Ota H, Aonuma K. Prognostic impact of supraventricular premature complexes in community-based health checkups: the Ibaraki Prefectural Health Study. Eur Heart J. 2015. Jan 14;36(3):170–8. doi: 10.1093/eurheartj/ehu407 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

