Modular cytosine base editing promotes epigenomic and genomic modifications
- PMID: 37994786
- PMCID: PMC10810192
- DOI: 10.1093/nar/gkad1118
Modular cytosine base editing promotes epigenomic and genomic modifications
Abstract
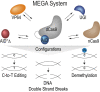
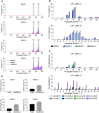
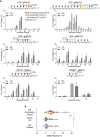
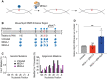
Prokaryotic and eukaryotic adaptive immunity differ considerably. Yet, their fundamental mechanisms of gene editing via Cas9 and activation-induced deaminase (AID), respectively, can be conveniently complimentary. Cas9 is an RNA targeted dual nuclease expressed in several bacterial species. AID is a cytosine deaminase expressed in germinal centre B cells to mediate genomic antibody diversification. AID can also mediate epigenomic reprogramming via active DNA demethylation. It is known that sequence motifs, nucleic acid structures, and associated co-factors affect AID activity. But despite repeated attempts, deciphering AID's intrinsic catalytic activities and harnessing its targeted recruitment to DNA is still intractable. Even recent cytosine base editors are unable to fully recapitulate AID's genomic and epigenomic editing properties. Here, we describe the first instance of a modular AID-based editor that recapitulates the full spectrum of genomic and epigenomic editing activity. Our 'Swiss army knife' toolbox will help better understand AID biology per se as well as improve targeted genomic and epigenomic editing.
© The Author(s) 2023. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

