The 5-HT7 receptor system as a treatment target for mood and anxiety disorders: A systematic review
- PMID: 37994803
- PMCID: PMC10714716
- DOI: 10.1177/02698811231211228
The 5-HT7 receptor system as a treatment target for mood and anxiety disorders: A systematic review
Abstract
Background: Preclinical animal and preliminary human studies indicate that 5-HT7 antagonists have the potential as a new treatment approach for mood and anxiety disorders. In this systematic review, we aimed to review the relationship between the 5-HT7 receptor system and mood and anxiety disorders, and to explore the pharmacology and therapeutic potential of medications that target the 5-HT7 receptor for their treatment.
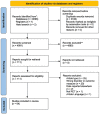
Methods: Medline, Cochrane Library, EMBASE, PsycINFO databases, the National Institute of Health website Clinicaltrials.gov, controlled-trials.com, and relevant grey literature were used to search for original research articles, and reference lists of included articles were then hand searched.
Results: Sixty-four studies were included in the review: 52 animal studies and 12 human studies. Studies used a variety of preclinical paradigms and questionnaires to assess change in mood, and few studies examined sleep or cognition. Forty-four out of 47 (44/47) preclinical 5-HT7 modulation studies identified potential antidepressant effects and 20/23 studies identified potential anxiolytic effects. In clinical studies, 5/7 identified potential antidepressant effects in major depressive disorder, 1/2 identified potential anxiolytic effects in generalized anxiety disorder, and 3/3 identified potential antidepressant effects in bipolar disorders.
Conclusion: While there is some evidence that the 5-HT7 receptor system may be a potential target for treating mood and anxiety disorders, many agents included in the review also bind to other receptors. Further research is needed using drugs that bind specifically to 5-HT7 receptors to examine treatment proof of concept further.
Keywords: 5-HT7; anxiety disorders; bipolar disorders; major depressive disorder; systematic review.
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AY declares honoraria for speaking from Astra Zeneca, Lundbeck, Eli Lilly, Sunovion; honoraria for consulting from Allergan, Livanova and Lundbeck, Sunovion, Janssen; and research grant support from Janssen. He is an editor for Journal of Psychopharmacology and Deputy Editor, BJPsych Open. PS reports personal fees and nonfinancial support from Frontiers in Psychiatry, personal fees from Allergan and a grant from H Lundbeck outside the submitted work. All authors report support from a grant from the Medical Research Council UK and nonfinancial support from Janssen Research and Development LLC during the conduct of the study. No other declarations of interest are reported.
Figures
References
-
- Adell A. (2010) Lu-AA21004, a multimodal serotonergic agent, for the potential treatment of depression and anxiety. IDrugs 13: 900–910. - PubMed
-
- Armijo-Olivo S, Stiles CR, Hagen NA, et al. (2012) Assessment of study quality for systematic reviews: A comparison of the Cochrane Collaboration Risk of Bias Tool and the Effective Public Health Practice Project Quality Assessment Tool: Methodological research. J Eval Clin Pract 18: 12–18. DOI: 10.1111/j.1365-2753.2010.01516.x. - DOI - PubMed