The Brain-Heart Axis: Neuroinflammatory Interactions in Cardiovascular Disease
- PMID: 37994952
- PMCID: PMC10908342
- DOI: 10.1007/s11886-023-01990-8
The Brain-Heart Axis: Neuroinflammatory Interactions in Cardiovascular Disease
Abstract
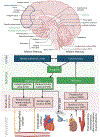
Purpose of review: The role of neuroimmune modulation and inflammation in cardiovascular disease has been historically underappreciated. Physiological connections between the heart and brain, termed the heart-brain axis (HBA), are bidirectional, occur through a complex network of autonomic nerves/hormones and cytokines, and play important roles in common disorders.
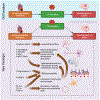
Recent findings: At the molecular level, advances in the past two decades reveal complex crosstalk mediated by the sympathetic and parasympathetic nervous systems, the renin-angiotensin aldosterone and hypothalamus-pituitary axes, microRNA, and cytokines. Afferent pathways amplify proinflammatory signals via the hypothalamus and brainstem to the periphery, promoting neurogenic inflammation. At the organ level, while stress-mediated cardiomyopathy is the prototypical disorder of the HBA, cardiac dysfunction can result from a myriad of neurologic insults including stroke and spinal injury. Atrial fibrillation is not necessarily a causative factor for cardioembolic stroke, but a manifestation of an abnormal atrial substrate, which can lead to the development of stroke independent of AF. Central and peripheral neurogenic proinflammatory factors have major roles in the HBA, manifesting as complex bi-directional relationships in common conditions such as stroke, arrhythmia, and cardiomyopathy.
Keywords: Arrhythmia-related stroke; Cardiometabolic syndrome; Cardiovascular neurogenic aging; Heart brain axis; Neuroinflammatory interactions; Stress cardiomyopathy.
© 2023. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

