TRP Channels in Stroke
- PMID: 37995056
- PMCID: PMC11306852
- DOI: 10.1007/s12264-023-01151-5
TRP Channels in Stroke
Abstract
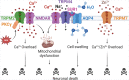
Ischemic stroke is a devastating disease that affects millions of patients worldwide. Unfortunately, there are no effective medications for mitigating brain injury after ischemic stroke. TRP channels are evolutionally ancient biosensors that detect external stimuli as well as tissue or cellular injury. To date, many members of the TRP superfamily have been reported to contribute to ischemic brain injury, including the TRPC subfamily (1, 3, 4, 5, 6, 7), TRPV subfamily (1, 2, 3, 4) and TRPM subfamily (2, 4, 7). These TRP channels share structural similarities but have distinct channel functions and properties. Their activation during ischemic stroke can be beneficial, detrimental, or even both. In this review, we focus on discussing the interesting features of stroke-related TRP channels and summarizing the underlying cellular and molecular mechanisms responsible for their involvement in ischemic brain injury.
Keywords: Blood-brain barrier; Glial activation; Immune cell infiltration; Neuronal death; Stroke; TRP channels.
© 2023. Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

