Lack of antiviral activity of probenecid in vitro and in Syrian golden hamsters
- PMID: 37995258
- PMCID: PMC10761260
- DOI: 10.1093/jac/dkad362
Lack of antiviral activity of probenecid in vitro and in Syrian golden hamsters
Abstract
Objectives: Antiviral interventions are required to complement vaccination programmes and reduce the global burden of COVID-19. Prior to initiation of large-scale clinical trials, robust preclinical data to support candidate plausibility are required. This work sought to further investigate the putative antiviral activity of probenecid against SARS-CoV-2.
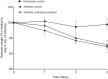
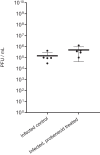
Methods: Vero E6 cells were preincubated with probenecid, or control media for 2 h before infection (SARS-CoV-2/Human/Liverpool/REMRQ0001/2020). Probenecid or control media was reapplied, plates reincubated and cytopathic activity quantified by spectrophotometry after 48 h. In vitro human airway epithelial cell (HAEC) assays were performed for probenecid against SARS-CoV-2-VoC-B.1.1.7 (hCoV-19/Belgium/rega-12211513/2020; EPI_ISL_791333, 2020-12-21) using an optimized cell model for antiviral testing. Syrian golden hamsters were intranasally inoculated (SARS-CoV-2 Delta B.1.617.2) 24 h prior to treatment with probenecid or vehicle for four twice-daily doses.
Results: No observable antiviral activity for probenecid was evident in Vero E6 or HAEC assays. No reduction in total or subgenomic RNA was observed in terminal lung samples (P > 0.05) from hamsters. Body weight of uninfected hamsters remained stable whereas both probenecid- and vehicle-treated infected hamsters lost body weight (P > 0.5).
Conclusions: These data do not support probenecid as a SARS-CoV-2 antiviral drug.
© The Author(s) 2023. Published by Oxford University Press on behalf of British Society for Antimicrobial Chemotherapy.
Figures


References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

