DeepFold: enhancing protein structure prediction through optimized loss functions, improved template features, and re-optimized energy function
- PMID: 37995286
- PMCID: PMC10699847
- DOI: 10.1093/bioinformatics/btad712
DeepFold: enhancing protein structure prediction through optimized loss functions, improved template features, and re-optimized energy function
Erratum in
-
Correction to: DeepFold: enhancing protein structure prediction through optimized loss functions, improved template features, and re-optimized energy function.Bioinformatics. 2023 Dec 1;39(12):btad762. doi: 10.1093/bioinformatics/btad762. Bioinformatics. 2023. PMID: 38140709 Free PMC article. No abstract available.
Abstract
Motivation: Predicting protein structures with high accuracy is a critical challenge for the broad community of life sciences and industry. Despite progress made by deep neural networks like AlphaFold2, there is a need for further improvements in the quality of detailed structures, such as side-chains, along with protein backbone structures.
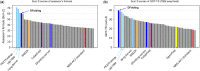
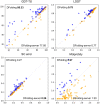
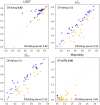
Results: Building upon the successes of AlphaFold2, the modifications we made include changing the losses of side-chain torsion angles and frame aligned point error, adding loss functions for side chain confidence and secondary structure prediction, and replacing template feature generation with a new alignment method based on conditional random fields. We also performed re-optimization by conformational space annealing using a molecular mechanics energy function which integrates the potential energies obtained from distogram and side-chain prediction. In the CASP15 blind test for single protein and domain modeling (109 domains), DeepFold ranked fourth among 132 groups with improvements in the details of the structure in terms of backbone, side-chain, and Molprobity. In terms of protein backbone accuracy, DeepFold achieved a median GDT-TS score of 88.64 compared with 85.88 of AlphaFold2. For TBM-easy/hard targets, DeepFold ranked at the top based on Z-scores for GDT-TS. This shows its practical value to the structural biology community, which demands highly accurate structures. In addition, a thorough analysis of 55 domains from 39 targets with publicly available structures indicates that DeepFold shows superior side-chain accuracy and Molprobity scores among the top-performing groups.
Availability and implementation: DeepFold tools are open-source software available at https://github.com/newtonjoo/deepfold.
© The Author(s) 2023. Published by Oxford University Press.
Conflict of interest statement
None declared.
Figures



References
-
- Anfinsen CB. Principles that govern the folding of protein chains. Science 1973;181:223–30. - PubMed

