Real-World Outcomes of Trastuzumab Deruxtecan in Patients With HER2+ Metastatic Breast Cancer: The DE-REAL Study
- PMID: 37995313
- PMCID: PMC10994255
- DOI: 10.1093/oncolo/oyad308
Real-World Outcomes of Trastuzumab Deruxtecan in Patients With HER2+ Metastatic Breast Cancer: The DE-REAL Study
Abstract
Background: Trastuzumab deruxtecan (T-DXd) demonstrated unprecedented efficacy in patients with pretreated HER2+ metastatic breast cancer (mBC). However, few data are available about its efficacy in routine clinical practice. In this multicenter retrospective study, we examined effectiveness and safety of T-DXd in a real-world population.
Methods: Clinico-pathological information about patients with HER2+ mBC who received T-DXd were collected from 12 Italian hospitals. HER2 status was determined locally. Patients who received at least one administration of T-DXd, as any therapy line for advanced disease were included in the analysis. The primary endpoint was real-word PFS (rwPFS).
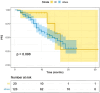
Results: One hundred and forty-three patients were included. Median age was 66 (range: 37-90), and 4 men were included. Hormone receptor (HR) status was positive in 108 (75%) patients and negative in 35(25%). T-DXd was administered as first, second, third, or subsequent lines in 4 (3%), 16 (11%), 42 (29%), and 81 (57%) patients, respectively. Among 123 patients with measurable disease, the ORR was 68%, and the DCR was 93% (9 CRs, 74 PRs, and 30 SD). Nine (7%) patients had a primary resistance to T-DXd. With a median follow-up of 12 months, the median rwPFS was 16 months. RwPFS was 84%, 59%, and 39% at 6, 12, and 18 months, respectively. A favorable trend in rwPFS was reported in patients receiving T-DXd as I/II line versus further lines (17 vs. 15 months; P = .098). Any-grade toxicity was registered in 84 patients (59%). Most common adverse events (AEs) reported were nausea (33%), neutropenia (21%), and asthenia (21%). Liver toxicity and diarrhea were uncommon (5% and 1%). Severe toxicities was registered in 18% of patients, and the most frequent were neutropenia, nausea/vomiting, and ILD observed in 15, 2, and 3 patients. AEs led to dose reduction in 37 patients (26%). Dose reduction and AEs do not affect patients' response and survival outcomes.
Conclusions: Efficacy and safety of T-DXd were confirmed in an unselected real-world population of HER2+ mBC. These results are consistent with the results of known findings, and no new safety concerns were reported.
Keywords: DE-REAL study; HER2+; breast cancer; trastuzumab deruxtecan.
© The Author(s) 2023. Published by Oxford University Press.
Conflict of interest statement
Andrae Bottticelli has advisory and consulting role for Roche, MSD, Novartis, Pfizer, Lilly, Amgen, BMS, Gliead, Sofos, Daichii, and AstraZeneca. Roberta Capuro reported advisory roles with Roche, Lilly, Novartis, MSD, Gilead, Daiichi Sankyo, and Pierre-Fabre. Simone Scagnoli reported speaker fees from Novartis, Pfizer, Roche, Lilly, BMS, and MSD. Simona Pisegna reported speaker honoraria from Novartis, Lilly, Pfizer, Roche, AstraZeneca, and Gilead. Michelino De Laurentiis reported consulting for Astrazeneca, Amgen, Celgene, Daiichi Sankyo, Eisai, Eli Lilly, Exact-Science, Gilead, MSD, Novartis, Pfizer, Pierre Fabre, Roche, and Seagen. Giuseppe Curigliano reported consultant fee for BMS, Roche, Pfizer, Novartis, Lilly, AstraZeneca, Daichii Sankyo, Merck, Seagen, Ellipsis, and Gilead, and a grant from Merck. Francesco Pantano reported advisory board for Gilead, AstraZeneca, Daiichi Sankyo, and Novartis. Antonella Palazzo reported advisory board/invited speaker for AstraZeneca, Daichii-Sankyo, Novartis, Pfizer, Eli Lilly, and MSD. Claudio Vernieri reported advisory role for Eli Lilly, Novartis, Pfizer, and Daiichi Sankyo; honoraria as a speaker for Eli Lilly, Novartis, Pfizer, Daiichi Sankyo, Istituto Gentili, Accademia di Medicina; and research grants from Roche. Michela Palleschi reported advisory board member for Novartis and Lilly. Giuliana D’Auria reported advisory role for Amgen, Pfizer, Lilly, Novartis, and Eisai. Agnese Fabbri reported advisory and speaker fees from Lilly, Pfizer, Roche, Novartis, and Gilead. Paolo Marchetti has/had a consultant/advisory role for BMS, Roche, Genentech, MSD, Novartis, Amgen, Merck Serono, Pierre-Fabre, and Incyte. Daniele Santini reported advisory board for Angen, Janssen, Astellas, Bayer, Servier, Novartis, MSD. Merck, Pfizer, and Ipsen. Alessandra Fabi reported advisory board for Roche, Pfizer, Novartis, Gilead, Sophos, Seagen, AstraZeneca, Lilly, Pierre Fabre, Exact Science. The other authors indicated no financial relationships.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

