S. aureus drives itch and scratch-induced skin damage through a V8 protease-PAR1 axis
- PMID: 37995657
- PMCID: PMC10669764
- DOI: 10.1016/j.cell.2023.10.019
S. aureus drives itch and scratch-induced skin damage through a V8 protease-PAR1 axis
Abstract
Itch is an unpleasant sensation that evokes a desire to scratch. The skin barrier is constantly exposed to microbes and their products. However, the role of microbes in itch generation is unknown. Here, we show that Staphylococcus aureus, a bacterial pathogen associated with itchy skin diseases, directly activates pruriceptor sensory neurons to drive itch. Epicutaneous S. aureus exposure causes robust itch and scratch-induced damage. By testing multiple isogenic bacterial mutants for virulence factors, we identify the S. aureus serine protease V8 as a critical mediator in evoking spontaneous itch and alloknesis. V8 cleaves proteinase-activated receptor 1 (PAR1) on mouse and human sensory neurons. Targeting PAR1 through genetic deficiency, small interfering RNA (siRNA) knockdown, or pharmacological blockade decreases itch and skin damage caused by V8 and S. aureus exposure. Thus, we identify a mechanism of action for a pruritogenic bacterial factor and demonstrate the potential of inhibiting V8-PAR1 signaling to treat itch.
Keywords: PAR1; Staphylococcus aureus; V8 protease; itch; microbe; proteinase-activated receptor; pruriceptor.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests I.M.C. serves on the SAB of GSK Pharmaceuticals. Provisional patent application serial no. 63/438,668, of which some co-authors are inventors, was filed based on these findings.
Figures
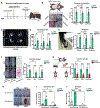
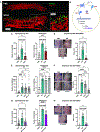
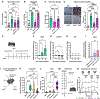

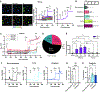
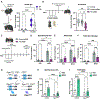
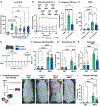
Comment in
-
Better than being aPARt: S. aureus itches to get close to sensory neurons.Cell Host Microbe. 2024 Jan 10;32(1):3-4. doi: 10.1016/j.chom.2023.12.011. Cell Host Microbe. 2024. PMID: 38211562
Comment on
-
Exploring the binding effect and mechanism of glycyrrhizin to ovomucin by combining spectroscopic analysis and molecular docking.Int J Biol Macromol. 2023 Aug 1;245:125535. doi: 10.1016/j.ijbiomac.2023.125535. Epub 2023 Jun 24. Int J Biol Macromol. 2023. PMID: 37356685
References
-
- Campione E, Lanna C, Diluvio L, Cannizzaro MV, Grelli S, Galluzzo M, Talamonti M, Annicchiarico-Petruzzelli M, Mancini M, Melino G, et al. (2020). Skin immunity and its dysregulation in atopic dermatitis, hidradenitis suppurativa and vitiligo. Cell Cycle 19, 257–267. 10.1080/15384101.2019.1707455. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 AI052453/AI/NIAID NIH HHS/United States
- R01 NS111929/NS/NINDS NIH HHS/United States
- U01 AI152038/AI/NIAID NIH HHS/United States
- R01 NS065926/NS/NINDS NIH HHS/United States
- R21 AG075419/AG/NIA NIH HHS/United States
- UM1 AI151958/AI/NIAID NIH HHS/United States
- R01 AI153185/AI/NIAID NIH HHS/United States
- R01 NS102161/NS/NINDS NIH HHS/United States
- P30 CA006516/CA/NCI NIH HHS/United States
- T32 AI007036/AI/NIAID NIH HHS/United States
- R01 AI168005/AI/NIAID NIH HHS/United States
- R01 AR076082/AR/NIAMS NIH HHS/United States
- F32 AI172080/AI/NIAID NIH HHS/United States
- T32 AI049928/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

