Lidocaine induces apoptosis in head and neck squamous cell carcinoma through activation of bitter taste receptor T2R14
- PMID: 37995679
- PMCID: PMC10842818
- DOI: 10.1016/j.celrep.2023.113437
Lidocaine induces apoptosis in head and neck squamous cell carcinoma through activation of bitter taste receptor T2R14
Abstract
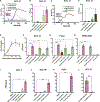
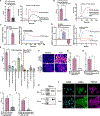
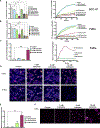
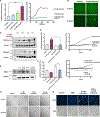
Head and neck squamous cell carcinomas (HNSCCs) have high mortality and significant treatment-related morbidity. It is vital to discover effective, minimally invasive therapies that improve survival and quality of life. Bitter taste receptors (T2Rs) are expressed in HNSCCs, and T2R activation can induce apoptosis. Lidocaine is a local anesthetic that also activates bitter taste receptor 14 (T2R14). Lidocaine has some anti-cancer effects, but the mechanisms are unclear. Here, we find that lidocaine causes intracellular Ca2+ mobilization through activation of T2R14 in HNSCC cells. T2R14 activation with lidocaine depolarizes mitochondria, inhibits proliferation, and induces apoptosis. Concomitant with mitochondrial Ca2+ influx, ROS production causes T2R14-dependent accumulation of poly-ubiquitinated proteins, suggesting that proteasome inhibition contributes to T2R14-induced apoptosis. Lidocaine may have therapeutic potential in HNSCCs as a topical gel or intratumor injection. In addition, we find that HPV-associated (HPV+) HNSCCs are associated with increased TAS2R14 expression. Lidocaine treatment may benefit these patients, warranting future clinical studies.
Keywords: CP: Cancer; G-protein-coupled receptor; HPV+; anesthetic; apoptosis; bitter agonist; calcium; chemosensory receptor; cyclic-AMP; ubiquitin-proteasome system.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


Similar articles
-
Pseudomonas aeruginosa metabolite 3-oxo-C12HSL induces apoptosis through T2R14 and the mitochondrial calcium uniporter.bioRxiv [Preprint]. 2024 Oct 29:2024.10.24.620094. doi: 10.1101/2024.10.24.620094. bioRxiv. 2024. PMID: 39553967 Free PMC article. Preprint.
-
T2R bitter taste receptors regulate apoptosis and may be associated with survival in head and neck squamous cell carcinoma.Mol Oncol. 2022 Apr;16(7):1474-1492. doi: 10.1002/1878-0261.13131. Epub 2021 Dec 14. Mol Oncol. 2022. PMID: 34717036 Free PMC article.
-
Highly conserved intracellular H208 residue influences agonist selectivity in bitter taste receptor T2R14.Biochim Biophys Acta Biomembr. 2019 Dec 1;1861(12):183057. doi: 10.1016/j.bbamem.2019.183057. Epub 2019 Sep 5. Biochim Biophys Acta Biomembr. 2019. PMID: 31493373
-
Human Papillomavirus Associated Cancers of the Head and Neck: An Australian Perspective.Head Neck Pathol. 2017 Sep;11(3):377-384. doi: 10.1007/s12105-017-0780-7. Epub 2017 Feb 7. Head Neck Pathol. 2017. PMID: 28176136 Free PMC article. Review.
-
Constitutive activity of bitter taste receptors (T2Rs).Adv Pharmacol. 2014;70:303-26. doi: 10.1016/B978-0-12-417197-8.00010-9. Adv Pharmacol. 2014. PMID: 24931200 Review.
Cited by
-
Effects of Akt Activator SC79 on Human M0 Macrophage Phagocytosis and Cytokine Production.Cells. 2024 May 24;13(11):902. doi: 10.3390/cells13110902. Cells. 2024. PMID: 38891035 Free PMC article.
-
GLUT1 inhibitor BAY-876 induces apoptosis and enhances anti-cancer effects of bitter receptor agonists in head and neck squamous carcinoma cells.Cell Death Discov. 2024 Jul 25;10(1):339. doi: 10.1038/s41420-024-02106-z. Cell Death Discov. 2024. PMID: 39060287 Free PMC article.
-
Lidocaine as a Potential Therapeutic Agent in Colorectal Cancer: A Study of Gene Expression and Prognosis.Onco Targets Ther. 2025 Jun 18;18:737-749. doi: 10.2147/OTT.S505753. eCollection 2025. Onco Targets Ther. 2025. PMID: 40547064 Free PMC article.
-
Hops bitter β-acids have antibacterial effects against sinonasal Staphylococcus aureus but also induce sinonasal cilia and mitochondrial dysfunction.Int Forum Allergy Rhinol. 2025 Mar;15(3):287-302. doi: 10.1002/alr.23487. Epub 2024 Nov 13. Int Forum Allergy Rhinol. 2025. PMID: 39533961 Free PMC article.
-
Reassessing lidocaine as an electroporation sensitizer in vitro.Sci Rep. 2025 Jul 15;15(1):25593. doi: 10.1038/s41598-025-11695-3. Sci Rep. 2025. PMID: 40665133 Free PMC article.
References
-
- Solomon B, Young RJ, and Rischin D (2018). Head and neck squamous cell carcinoma: Genomics and emerging biomarkers for immunomodulatory cancer treatments. Semin. Cancer Biol 52, 228–240. - PubMed
-
- Kawakita D, and Matsuo K (2017). Alcohol and head and neck cancer. Cancer Metastasis Rev. 36, 425–434. - PubMed
-
- Hannen EJM, and Riediger D (2004). The quantification of angiogenesis in relation to metastasis in oral cancer: a review. Int. J. Oral Maxillofac. Surg 33, 2–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

