IntS6 and the Integrator phosphatase module tune the efficiency of select premature transcription termination events
- PMID: 37995689
- PMCID: PMC10841813
- DOI: 10.1016/j.molcel.2023.10.035
IntS6 and the Integrator phosphatase module tune the efficiency of select premature transcription termination events
Abstract
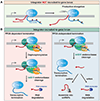
The metazoan-specific Integrator complex catalyzes 3' end processing of small nuclear RNAs (snRNAs) and premature termination that attenuates the transcription of many protein-coding genes. Integrator has RNA endonuclease and protein phosphatase activities, but it remains unclear if both are required for complex function. Here, we show IntS6 (Integrator subunit 6) over-expression blocks Integrator function at a subset of Drosophila protein-coding genes, although having no effect on snRNAs or attenuation of other loci. Over-expressed IntS6 titrates protein phosphatase 2A (PP2A) subunits, thereby only affecting gene loci where phosphatase activity is necessary for Integrator function. IntS6 functions analogous to a PP2A regulatory B subunit as over-expression of canonical B subunits, which do not bind Integrator, is also sufficient to inhibit Integrator activity. These results show that the phosphatase module is critical at only a subset of Integrator-regulated genes and point to PP2A recruitment as a tunable step that modulates transcription termination efficiency.
Keywords: 3′ end processing; INTAC; Integrator complex; Integrator subunit 6; PP2A; RNA polymerase II; promoter-proximal termination; protein phosphatase 2A; snRNA; transcription.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests J.E.W. serves as a consultant for Laronde.
Figures






Similar articles
-
Integrator Recruits Protein Phosphatase 2A to Prevent Pause Release and Facilitate Transcription Termination.Mol Cell. 2020 Oct 15;80(2):345-358.e9. doi: 10.1016/j.molcel.2020.08.016. Epub 2020 Sep 22. Mol Cell. 2020. PMID: 32966759 Free PMC article.
-
Structural basis of Integrator-dependent RNA polymerase II termination.Nature. 2024 May;629(8010):219-227. doi: 10.1038/s41586-024-07269-4. Epub 2024 Apr 3. Nature. 2024. PMID: 38570683 Free PMC article.
-
Genomic regulation of transcription and RNA processing by the multitasking Integrator complex.Nat Rev Mol Cell Biol. 2023 Mar;24(3):204-220. doi: 10.1038/s41580-022-00534-2. Epub 2022 Sep 30. Nat Rev Mol Cell Biol. 2023. PMID: 36180603 Free PMC article. Review.
-
A subset of Drosophila integrator proteins is essential for efficient U7 snRNA and spliceosomal snRNA 3'-end formation.Mol Cell Biol. 2011 Jan;31(2):328-41. doi: 10.1128/MCB.00943-10. Epub 2010 Nov 15. Mol Cell Biol. 2011. PMID: 21078872 Free PMC article.
-
Regulation of snRNA gene expression by the Drosophila melanogaster small nuclear RNA activating protein complex (DmSNAPc).Crit Rev Biochem Mol Biol. 2011 Feb;46(1):11-26. doi: 10.3109/10409238.2010.518136. Epub 2010 Oct 6. Crit Rev Biochem Mol Biol. 2011. PMID: 20925482 Review.
Cited by
-
Redundant pathways for removal of defective RNA polymerase II complexes at a promoter-proximal pause checkpoint.Mol Cell. 2024 Dec 19;84(24):4790-4807.e11. doi: 10.1016/j.molcel.2024.10.012. Epub 2024 Nov 5. Mol Cell. 2024. PMID: 39504960
-
Construction of a Prognostic Model Using RNA Processing Factor Genes and the Key Role of NSUN6 in Glioma Outcomes.J Cell Mol Med. 2025 Jun;29(12):e70668. doi: 10.1111/jcmm.70668. J Cell Mol Med. 2025. PMID: 40561176 Free PMC article.
-
Catalytic-independent functions of the Integrator-PP2A complex (INTAC) confer sensitivity to BET inhibition.Nat Chem Biol. 2025 Jun;21(6):959-970. doi: 10.1038/s41589-024-01807-x. Epub 2025 Jan 14. Nat Chem Biol. 2025. PMID: 39809894
-
The PNUTS phosphatase complex controls transcription pause release.Mol Cell. 2024 Dec 19;84(24):4843-4861.e8. doi: 10.1016/j.molcel.2024.10.045. Epub 2024 Nov 26. Mol Cell. 2024. PMID: 39603239
-
Multiple Forms and Functions of Premature Termination by RNA Polymerase II.J Mol Biol. 2025 Jan 1;437(1):168743. doi: 10.1016/j.jmb.2024.168743. Epub 2024 Aug 9. J Mol Biol. 2025. PMID: 39127140 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

