Inserting a Neuropixels probe into awake monkey cortex: two probes, two methods
- PMID: 37995854
- PMCID: PMC10843751
- DOI: 10.1016/j.jneumeth.2023.110016
Inserting a Neuropixels probe into awake monkey cortex: two probes, two methods
Abstract
Background: Neuropixels probes have revolutionized neurophysiological studies in the rodent, but inserting these probes through the much thicker primate dura remains a challenge.
New methods: Here we describe two methods we have developed for the insertion of two types of Neuropixels probes acutely into the awake macaque monkey cortex. For the fine rodent probe (Neuropixels 1.0, IMEC), which is unable to pierce native primate dura, we developed a dural-eyelet method to insert the probe repeatedly without breakage. For the thicker short NHP probe (Neuropixels NP1010), we developed an artificial dura system to insert the probe.
Results and comparison with existing methods: We have now conducted successful experiments in 3 animals across 7 recording chambers with the procedures described here and have achieved recordings with similar yields over several months in each case.
Conclusion: We hope that our hardware, surgical preparation, methods for insertion and methods for removal of broken probe parts are of value to primate physiologists everywhere.
Keywords: Chronic recording chamber; High-density silicon probe; Multi-contact linear probes; Nonhuman primate neurophysiology.
Copyright © 2023 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no competing interests.
Figures



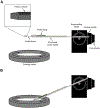

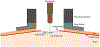

Update of
-
Inserting a Neuropixels probe into awake monkey cortex: two probes, two methods.bioRxiv [Preprint]. 2023 Jun 29:2023.06.26.546631. doi: 10.1101/2023.06.26.546631. bioRxiv. 2023. Update in: J Neurosci Methods. 2024 Feb;402:110016. doi: 10.1016/j.jneumeth.2023.110016. PMID: 37425930 Free PMC article. Updated. Preprint.
References
-
- Barnett AC, Jones JA, Lee Y, and Moore JZ, 2016. Compliant Needle Vibration Cutting of Soft Tissue. J. Manuf. Sci. Eng November 2016; 138(11): 111011.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

