Engineering therapeutic monoclonal antibodies
- PMID: 37995859
- PMCID: PMC11437839
- DOI: 10.1016/j.jaci.2023.11.018
Engineering therapeutic monoclonal antibodies
Abstract
The use of human antibodies as biologic therapeutics has revolutionized patient care throughout fields of medicine. As our understanding of the many roles antibodies play within our natural immune responses continues to advance, so will the number of therapeutic indications for which an mAb will be developed. The great breadth of function, long half-life, and modular structure allow for nearly limitless therapeutic possibilities. Human antibodies can be rationally engineered to enhance their desired immune functions and eliminate those that may result in unwanted effects. Antibody therapeutics now often start with fully human variable regions, either acquired from genetically engineered humanized mice or from the actual human B cells. These variable genes can be further engineered by widely used methods for optimization of their specificity through affinity maturation, random mutagenesis, targeted mutagenesis, and use of in silico approaches. Antibody isotype selection and deliberate mutations are also used to improve efficacy and tolerability by purposeful fine-tuning of their immune effector functions. Finally, improvements directed at binding to the neonatal Fc receptor can endow therapeutic antibodies with unbelievable extensions in their circulating half-life. The future of engineered antibody therapeutics is bright, with the global mAb market projected to exhibit compound annual growth, forecasted to reach a revenue of nearly half a trillion dollars in 2030.
Keywords: Allergy; IgE; antibody engineering; immunology; mAb; therapeutic antibody.
Copyright © 2023 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Author conflicts of interest:
SAS receives royalties for intellectual property licenses with InBio Inc. and consulting fees from IgGenix Inc. SAS is an inventor on a patent entitled “Generation of human allergen- and helminth-specific IgE monoclonal antibodies for diagnostic and therapeutic use” (U.S. patent no. 11709167), with royalties paid and on a pending patent entitled “Generation of Human Peanut Allergen-Specific IgE Monoclonal Antibodies for Diagnostic and Therapeutic Use” (PCT/US2022/019503), with royalties paid. BWS is a co-founder and principle at Turkey Creek Biotechnology (TCB). TCB was not involved in this work.
Figures

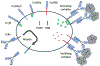

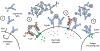
References
-
- Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. 1975. J Immunol. 2005. Mar 1;174(5):2453–5. - PubMed
-
- Yu RJ, Krantz MS, Phillips EJ, Stone CA Jr. Emerging Causes of Drug-Induced Anaphylaxis: A Review of Anaphylaxis-Associated Reports in the FDA Adverse Event Reporting System (FAERS). J Allergy Clin Immunol Pract. 2021. Feb;9(2):819–829.e2. doi: 10.1016/j.jaip.2020.09.021. Epub 2020 Sep 28.. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

