Staging by Thoracoscopy in potentially radically treatable Lung Cancer associated with Minimal Pleural Effusion (STRATIFY): protocol of a prospective, multicentre, observational study
- PMID: 37996118
- PMCID: PMC10668291
- DOI: 10.1136/bmjresp-2023-001771
Staging by Thoracoscopy in potentially radically treatable Lung Cancer associated with Minimal Pleural Effusion (STRATIFY): protocol of a prospective, multicentre, observational study
Abstract
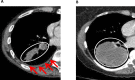
Introduction: Recurrence rate following radical therapy for lung cancer remains high, potentially reflecting occult metastatic disease, and better staging tools are required. Minimal pleural effusion (mini-PE) is associated with particularly high recurrence risk and is defined as an ipsilateral pleural collection (<1/3 hemithorax on chest radiograph), which is either too small to safely aspirate fluid for cytology using a needle, or from which fluid cytology is negative. Thoracoscopy (local anaesthetic thoracoscopy (LAT) or video-assisted thoracoscopic surgery (VATS)) is the gold-standard diagnostic test for pleural malignancy in patients with larger symptomatic effusions. Staging by Thoracoscopy in potentially radically treatable Lung Cancer associated with Minimal Pleural Effusion (STRATIFY) will prospectively evaluate thoracoscopic staging in lung cancer associated-mini-PE for the first time.
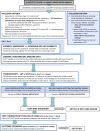
Methods and analysis: STRATIFY is a prospective multicentre observational study. Recruitment opened in January 2020. The primary objective is to determine the prevalence of detectable occult pleural metastases (OPM). Secondary objectives include assessment of technical feasibility and safety, and the impact of thoracoscopy results on treatment plans, overall survival and recurrence free survival. Inclusion criteria are (1) suspected/confirmed stages I-III lung cancer, (2) mini-PE, (3) Performance Status 0-2 (4), radical treatment feasible if OPM excluded, (5) ≥16 years old and (6) informed consent. Exclusion criteria are any metastatic disease or contraindication to the chosen thoracoscopy method (LAT/VATS). All patients have LAT or VATS within 7 (±5) days of registration, with results returned to lung cancer teams for treatment planning. Following an interim analysis, the sample size was reduced from 96 to 50, based on a lower-than-expected OPM rate. An MRI substudy was removed in November 2022 due to pandemic-related site setup/recruitment delays. These also necessitated a no-cost recruitment extension until October 2023.
Ethics and dissemination: Protocol approved by the West of Scotland Research Ethics Committee (Ref: 19/WS/0093). Results will be published in peer-reviewed journals and presented at international meetings.
Trial registration number: ISRCTN13584097.
Keywords: Lung Cancer; Pleural Disease; Thoracic Surgery.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Maguire J, Khan I, McMenemin R, et al. SOCCAR: a randomised phase II trial comparing sequential versus concurrent chemotherapy and radical hypofractionated radiotherapy in patients with inoperable stage III non-small cell lung cancer and good performance status. European Journal of Cancer 2014;50:2939–49. 10.1016/j.ejca.2014.07.009 - DOI - PubMed
-
- Semrau S, Klautke G, Fietkau R. Baseline cardiopulmonary function as an independent Prognostic factor for survival of inoperable non-small-cell lung cancer after concurrent chemoradiotherapy: a single-center analysis of 161 cases. Int J Radiat Oncol Biol Phys 2011;79:96–104. 10.1016/j.ijrobp.2009.10.010 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
