Titanium particles in peri-implantitis: distribution, pathogenesis and prospects
- PMID: 37996420
- PMCID: PMC10667540
- DOI: 10.1038/s41368-023-00256-x
Titanium particles in peri-implantitis: distribution, pathogenesis and prospects
Abstract
Peri-implantitis is one of the most important biological complications in the field of oral implantology. Identifying the causative factors of peri-implant inflammation and osteolysis is crucial for the disease's prevention and treatment. The underlying risk factors and detailed pathogenesis of peri-implantitis remain to be elucidated. Titanium-based implants as the most widely used implant inevitably release titanium particles into the surrounding tissue. Notably, the concentration of titanium particles increases significantly at peri-implantitis sites, suggesting titanium particles as a potential risk factor for the condition. Previous studies have indicated that titanium particles can induce peripheral osteolysis and foster the development of aseptic osteoarthritis in orthopedic joint replacement. However, it remains unconfirmed whether this phenomenon also triggers inflammation and bone resorption in peri-implant tissues. This review summarizes the distribution of titanium particles around the implant, the potential roles in peri-implantitis and the prevalent prevention strategies, which expects to provide new directions for the study of the pathogenesis and treatment of peri-implantitis.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests. Part of the figures were created with biorender.com.
Figures
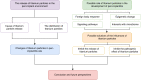
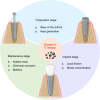
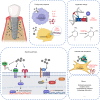

References
-
- Jepsen S, et al. Periodontal manifestations of systemic diseases and developmental and acquired conditions: Consensus report of workgroup 3 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018;89:S237–s248. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 82170992/National Natural Science Foundation of China (National Science Foundation of China)
- 82370990/National Natural Science Foundation of China (National Science Foundation of China)
- 82201051/National Natural Science Foundation of China (National Science Foundation of China)
- 2022M722742/China Postdoctoral Science Foundation
LinkOut - more resources
Full Text Sources

