Efficacy and quality of life for FOLFOX/bevacizumab +/- irinotecan in first-line metastatic colorectal cancer-final results of the AIO CHARTA trial
- PMID: 37996507
- PMCID: PMC10803799
- DOI: 10.1038/s41416-023-02496-4
Efficacy and quality of life for FOLFOX/bevacizumab +/- irinotecan in first-line metastatic colorectal cancer-final results of the AIO CHARTA trial
Abstract
Background: FOLFOXIRI plus bevacizumab has demonstrated benefits for metastatic colorectal cancer (mCRC) patients. However, challenges arise in its clinical implementation due to expected side effects and a lack of stratification criteria.
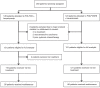
Methods: The AIO "CHARTA" trial randomised mCRC patients into clinical Group 1 (potentially resectable), 2 (unresectable/risk of rapid progression), or 3 (asymptomatic). They received FOLFOX/bevacizumab +/- irinotecan. The primary endpoint was the 9-month progression-free survival rate (PFSR@9). Secondary endpoints included efficacy in stratified groups, QoL, PFS, OS, ORR, secondary resection rate, and toxicity.
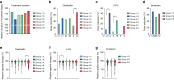
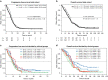
Results: The addition of irinotecan to FOLFOX/bevacizumab increased PFSR@9 from 56 to 67%, meeting the primary endpoint. The objective response rate was 61% vs. 69% (P = 0.21) and median PFS was 10.3 vs. 12 months (HR 0.83; P = 0.17). The PFS was (11.4 vs. 12.9 months; HR 0.83; P = 0.46) in potentially resectable patients, with a secondary resection rate of 37% vs. 51%. Moreover, Group 3 (asymptomatic) patients had a PFS of 11.1 vs. 16.1 months (HR 0.6; P = 0.14). The addition of irinotecan did not diminish QoL.
Conclusion: The CHARTA trial, along with other studies, confirms the efficacy and tolerability of FOLFOXIRI/bevacizumab as a first-line treatment for mCRC. Importantly, clinical stratification may lead to its implementation.
Trial registration: The trial was registered as NCT01321957.
© 2023. The Author(s).
Conflict of interest statement
HJS received honoraria and travel grants for lectures and advisory board meetings from Roche, Merk, Servier, AstraZeneca, Novartis, Sanofi, BMS. KB received honoraria for lectures and advisory board meetings Amgen, Roche, Sanofi-Aventis, Servier, Merck Serono. BH received personal fees and/or grants from Amgen, Bayer, Janssen, Lilly, Merck, Roche, Sanofi and Teva. AV received honoraria for lectures and advisory board meetings from Roche, Bayer, Sanofi, BMS, Lilly, Novartis, EISAI, AstraZeneca, Merck, Incyte, Medac, Ipsen, Servier, PierreFabre, MSD, BTG, Janssen and research funding from Servier. CB received institutional research grants and honoraria for lectures and advisory board meetings from Merck Darmstadt, BMS, Roche, Sanofi, Bayer Healthcare, AstraZeneca, Hexal, MSD, BioNTech, ODC and AOK Healthcare. AS received institutional research grants from Merck, BMS, Roche, Sanofi, Servier, German Cancer Aid and Federal Joint Committee and honoraria for lectures and advisory board meetings by Merck, Roche, Amgen, Lilly, Sanofi-Aventis, Servier, Bayer, BMS, MSD and Sirtex. The remaining authors declare no competing interests.
Figures

References
-
- Falcone A, Ricci S, Brunetti I, Pfanner E, Allegrini G, Barbara C, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol J Am Soc Clin Oncol. 2007;25:1670–6. doi: 10.1200/JCO.2006.09.0928. - DOI - PubMed
-
- Folprecht G, Hamann S, Schütte K, Trarbach T, Stoehlmacher-Williams J, Ehninger G. Dose escalating study of cetuximab and 5-FU/folinic acid (FA)/oxaliplatin/irinotecan (FOLFOXIRI) in first line therapy of patients with metastatic colorectal cancer. BMC Cancer. 2014;14:521. doi: 10.1186/1471-2407-14-521. - DOI - PMC - PubMed
-
- Fornaro L, Lonardi S, Masi G, Loupakis F, Bergamo F, Salvatore L, et al. FOLFOXIRI in combination with panitumumab as first-line treatment in quadruple wild-type (KRAS, NRAS, HRAS, BRAF) metastatic colorectal cancer patients: a phase II trial by the Gruppo Oncologico Nord Ovest (GONO) Ann Oncol J Eur Soc Med Oncol. 2013;24:2062–7. doi: 10.1093/annonc/mdt165. - DOI - PubMed
-
- Garufi C, Torsello A, Tumolo S, Ettorre GM, Zeuli M, Campanella C, et al. Cetuximab plus chronomodulated irinotecan, 5-fluorouracil, leucovorin and oxaliplatin as neoadjuvant chemotherapy in colorectal liver metastases: POCHER trial. Br J Cancer. 2010;103:1542–7. doi: 10.1038/sj.bjc.6605940. - DOI - PMC - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

