COVID-19 vaccine uptake among people with HIV: identifying characteristics associated with vaccine hesitancy
- PMID: 37996521
- PMCID: PMC10667522
- DOI: 10.1038/s41598-023-47106-8
COVID-19 vaccine uptake among people with HIV: identifying characteristics associated with vaccine hesitancy
Abstract
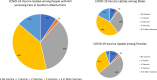
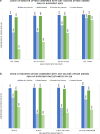
People with HIV (PWH) are at increased risk of COVID-19 infection. Both Canadian (NACI) and US (CDC) guidelines recommend that all PWH receive at least 2 doses of COVID-19 vaccine, and a booster. We examined vaccination uptake among PWH in Southern Alberta, Canada. Among adult PWH, we evaluated COVID-19 vaccination uptake between December 2020 and August 2022. Poisson regression models with robust variance (approximating log binomial models) estimated crude and adjusted prevalence ratios (aPR) and 95% confidence intervals (CI) for receiving (1) any vs. no vaccine, and (2) primary series with booster (≥ 3 vaccines) versus primary series without booster. Among 1885 PWH, 10% received no COVID-19 vaccinations, 37% < 3 vaccines and 54% received ≥ 3 vaccines. Females (vs. males) were less likely to receive a vaccine booster. Receiving no COVID-19 vaccines was associated with White ethnicity, unsuppressed HIV viral load (> 200 copies/mL), and using illegal substances. Factors associated with decreased booster uptake included being younger, Black (vs. White) ethnicity, substance use, lower educational attainment, and having an unsuppressed HIV viral load. COVID-19 booster uptake among PWH does not meet vaccine guidelines, and receipt of vaccines is unevenly distributed. Booster uptake is lowest among young females and marginalized individuals. Focused outreach is necessary to close this gap.
© 2023. The Author(s).
Conflict of interest statement
M.J.G. has in last five years received honoraria as an ad hoc member of national HIV advisory boards to Merck Gilead and ViiV. All other authors have no competing interests to disclose.
Figures
References
-
- Panel on Opportunistic Infections in Adults and Adolescents with HIV. Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV: Recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. 2022. http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf
-
- Centers for Disease Control and Prevention. Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Approved or Authorized in the United States. Interim Clin. Consid. Use COVID-19 Vaccines Curr. Approv. Auth. U. S. 2023. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-co...
-
- Jensen, C. P. R., Warshawsky, B., Krishnan, R., Wong, E., Zafack, J., Salvadori, M. I., Forbes, N., Abrams, E., Young, K., Tunis, M. C., Wilson, S., Harrison, R., Deeks, S. & On behalf of the National Advisory Committee on Immunization (NACI). COVID-19 vaccines: Canadian Immunization Guide 2023. (accessed Nov 1 2023) https://www.canada.ca/en/public-health/services/publications/healthy-liv...
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

