A dynamic sequence of visual processing initiated by gaze shifts
- PMID: 37996524
- PMCID: PMC11270614
- DOI: 10.1038/s41593-023-01481-7
A dynamic sequence of visual processing initiated by gaze shifts
Abstract
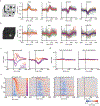
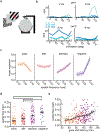
Animals move their head and eyes as they explore the visual scene. Neural correlates of these movements have been found in rodent primary visual cortex (V1), but their sources and computational roles are unclear. We addressed this by combining head and eye movement measurements with neural recordings in freely moving mice. V1 neurons responded primarily to gaze shifts, where head movements are accompanied by saccadic eye movements, rather than to head movements where compensatory eye movements stabilize gaze. A variety of activity patterns followed gaze shifts and together these formed a temporal sequence that was absent in darkness. Gaze-shift responses resembled those evoked by sequentially flashed stimuli, suggesting a large component corresponds to onset of new visual input. Notably, neurons responded in a sequence that matches their spatial frequency bias, consistent with coarse-to-fine processing. Recordings in freely gazing marmosets revealed a similar sequence following saccades, also aligned to spatial frequency preference. Our results demonstrate that active vision in both mice and marmosets consists of a dynamic temporal sequence of neural activity associated with visual sampling.
© 2023. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
COMPETING INTERESTS STATEMENT
The authors declare no competing interests.
Figures






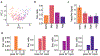



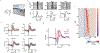

References
-
- Ahissar E & Arieli A Figuring space by time. Neuron 32, 185–201 (2001). - PubMed
-
- Gibson JJ The Ecological Approach to Visual Perception: Classic Edition. Psychology Press; (1979).
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

