Stathmin-2 loss leads to neurofilament-dependent axonal collapse driving motor and sensory denervation
- PMID: 37996528
- PMCID: PMC10842032
- DOI: 10.1038/s41593-023-01496-0
Stathmin-2 loss leads to neurofilament-dependent axonal collapse driving motor and sensory denervation
Abstract
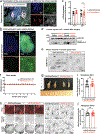
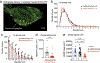
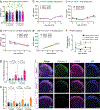
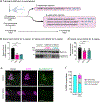
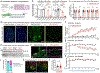
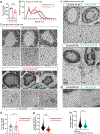
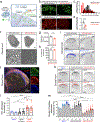
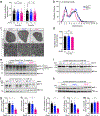
The mRNA transcript of the human STMN2 gene, encoding for stathmin-2 protein (also called SCG10), is profoundly impacted by TAR DNA-binding protein 43 (TDP-43) loss of function. The latter is a hallmark of several neurodegenerative diseases, including amyotrophic lateral sclerosis (ALS). Using a combination of approaches, including transient antisense oligonucleotide-mediated suppression, sustained shRNA-induced depletion in aging mice, and germline deletion, we show that stathmin-2 has an important role in the establishment and maintenance of neurofilament-dependent axoplasmic organization that is critical for preserving the caliber and conduction velocity of myelinated large-diameter axons. Persistent stathmin-2 loss in adult mice results in pathologies found in ALS, including reduced interneurofilament spacing, axonal caliber collapse that drives tearing within outer myelin layers, diminished conduction velocity, progressive motor and sensory deficits, and muscle denervation. These findings reinforce restoration of stathmin-2 as an attractive therapeutic approach for ALS and other TDP-43-dependent neurodegenerative diseases.
© 2023. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing Interests Statement
C.F.B., M.B.-H., K.L., P.J.-n., and F.R. are employees of Ionis Pharmaceuticals. D.W.C. is a consultant for Ionis Pharmaceuticals. Z.M. and D.W.C. have a relevant patent. C.L.-T serves on the scientific advisory board of SOLA Biosciences, Libra Therapeutics, Arbor Biotechnologies and Dewpoint Therapeutics and has received consultant fees from Mitsubishi Tanabe Pharma Holdings America and Applied Genetic Technologies Corporation. All other authors declare no competing interests.
Figures







References
-
- Rowland LP & Shneider NA Amyotrophic lateral sclerosis. N Engl J Med 344, 1688–1700 (2001). - PubMed
-
- Cleveland DW & Rothstein JD From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat Rev Neurosci 2, 806–819 (2001). - PubMed
-
- Fischer LR, et al. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol 185, 232–240 (2004). - PubMed
-
- Killian JM, Wilfong AA, Burnett L, Appel SH & Boland D Decremental motor responses to repetitive nerve stimulation in ALS. Muscle Nerve 17, 747–754 (1994). - PubMed
-
- Dengler R, et al. Amyotrophic lateral sclerosis: macro-EMG and twitch forces of single motor units. Muscle Nerve 13, 545–550 (1990). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

