Eph receptors and ephrins in cancer progression
- PMID: 37996538
- PMCID: PMC11015936
- DOI: 10.1038/s41568-023-00634-x
Eph receptors and ephrins in cancer progression
Abstract
Evidence implicating Eph receptor tyrosine kinases and their ephrin ligands (that together make up the 'Eph system') in cancer development and progression has been accumulating since the discovery of the first Eph receptor approximately 35 years ago. Advances in the past decade and a half have considerably increased the understanding of Eph receptor-ephrin signalling mechanisms in cancer and have uncovered intriguing new roles in cancer progression and drug resistance. This Review focuses mainly on these more recent developments. I provide an update on the different mechanisms of Eph receptor-ephrin-mediated cell-cell communication and cell autonomous signalling, as well as on the interplay of the Eph system with other signalling systems. I further discuss recent advances in elucidating how the Eph system controls tumour expansion, invasiveness and metastasis, supports cancer stem cells, and drives therapy resistance. In addition to functioning within cancer cells, the Eph system also mediates the reciprocal communication between cancer cells and cells of the tumour microenvironment. The involvement of the Eph system in tumour angiogenesis is well established, but recent findings also demonstrate roles in immune cells, cancer-associated fibroblasts and the extracellular matrix. Lastly, I discuss strategies under evaluation for therapeutic targeting of Eph receptors-ephrins in cancer and conclude with an outlook on promising future research directions.
© 2023. Springer Nature Limited.
Figures
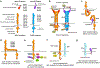
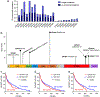
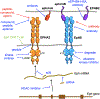
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

