Long-term HIF-1α stabilization reduces respiration, promotes mitophagy, and results in retinal cell death
- PMID: 37996657
- PMCID: PMC10667534
- DOI: 10.1038/s41598-023-47942-8
Long-term HIF-1α stabilization reduces respiration, promotes mitophagy, and results in retinal cell death
Abstract
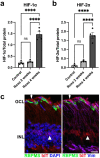
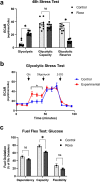
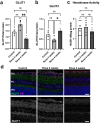
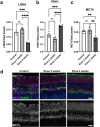
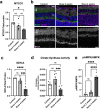
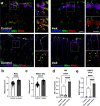
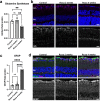
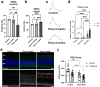
Ocular hypertension during glaucoma can lead to hypoxia, activation of the HIF transcription factors, and a metabolic shift toward glycolysis. This study aims to test whether chronic HIF activation and the attendant metabolic reprogramming can initiate glaucoma-associated pathology independently of ocular hypertension. HIF-1α stabilization was induced in mice for 2 and 4 weeks by inhibiting prolyl hydroxylases using the small molecule Roxadustat. HIF-1α stabilization and the expression of its downstream bioenergetic targets were investigated in the retina by immunofluorescence, capillary electrophoresis, and biochemical enzyme activity assays. Roxadustat dosing resulted in significant stabilization of HIF-1α in the retina by 4 weeks, and upregulation in glycolysis-associated proteins (GLUT3, PDK-1) and enzyme activity in both neurons and glia. Accordingly, succinate dehydrogenase, mitochondrial marker MTCO1, and citrate synthase activity were significantly decreased at 4 weeks, while mitophagy was significantly increased. TUNEL assay showed significant apoptosis of cells in the retina, and PERG amplitude was significantly decreased with 4 weeks of HIF-1α stabilization. A significant increase in AMPK activation and glial hypertrophy, concomitant with decreases in retinal ganglion cell function and inner retina cell death suggests that chronic HIF-1α stabilization alone is detrimental to retina metabolic homeostasis and cellular survival.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Oxidative Stress and Hypoxia Modify Mitochondrial Homeostasis During Glaucoma.Antioxid Redox Signal. 2021 Dec;35(16):1341-1357. doi: 10.1089/ars.2020.8180. Epub 2021 Apr 29. Antioxid Redox Signal. 2021. PMID: 33736457 Free PMC article.
-
Role of hypoxia-inducible factor-1α in preconditioning-induced protection of retinal ganglion cells in glaucoma.Mol Vis. 2013 Nov 23;19:2360-72. eCollection 2013. Mol Vis. 2013. PMID: 24319330 Free PMC article.
-
Hypoxia inducible factor-1α (HIF-1α) and some HIF-1 target genes are elevated in experimental glaucoma.J Mol Neurosci. 2010 Oct;42(2):183-91. doi: 10.1007/s12031-010-9343-z. Epub 2010 Mar 17. J Mol Neurosci. 2010. PMID: 20237864 Free PMC article.
-
Pharmacological regulation of HIF-1α, RGC death, and glaucoma.Curr Opin Pharmacol. 2024 Aug;77:102467. doi: 10.1016/j.coph.2024.102467. Epub 2024 Jun 18. Curr Opin Pharmacol. 2024. PMID: 38896924 Review.
-
Understanding glaucomatous damage: anatomical and functional data from ocular hypertensive rodent retinas.Prog Retin Eye Res. 2012 Jan;31(1):1-27. doi: 10.1016/j.preteyeres.2011.08.001. Epub 2011 Sep 21. Prog Retin Eye Res. 2012. PMID: 21946033 Review.
Cited by
-
Prolyl Hydroxylase Inhibitor-Mediated HIF Activation Drives Transcriptional Reprogramming in Retinal Pigment Epithelium: Relevance to Chronic Kidney Disease.Cells. 2025 Jul 21;14(14):1121. doi: 10.3390/cells14141121. Cells. 2025. PMID: 40710374 Free PMC article.
-
Identification of diagnostic biomarkers and dissecting immune microenvironment with crosstalk genes in the POAG and COVID-19 nexus.Sci Rep. 2025 Jul 12;15(1):25244. doi: 10.1038/s41598-025-10656-0. Sci Rep. 2025. PMID: 40652070 Free PMC article.
-
Ocular Inflammation and Oxidative Stress as a Result of Chronic Intermittent Hypoxia: A Rat Model of Sleep Apnea.Antioxidants (Basel). 2024 Jul 22;13(7):878. doi: 10.3390/antiox13070878. Antioxidants (Basel). 2024. PMID: 39061946 Free PMC article.
-
Protocol for the purification and culture of primary retinal ganglion cells and development of common pathological models.J Mol Histol. 2025 Aug 8;56(4):260. doi: 10.1007/s10735-025-10536-x. J Mol Histol. 2025. PMID: 40779178
-
The Identification of New Pharmacological Targets for the Treatment of Glaucoma: A Network Pharmacology Approach.Pharmaceuticals (Basel). 2024 Oct 5;17(10):1333. doi: 10.3390/ph17101333. Pharmaceuticals (Basel). 2024. PMID: 39458974 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

