Platelet and mitochondrial RNA is decreased in plasma-derived extracellular vesicles in women with preeclampsia-an exploratory study
- PMID: 37996819
- PMCID: PMC10666366
- DOI: 10.1186/s12916-023-03178-x
Platelet and mitochondrial RNA is decreased in plasma-derived extracellular vesicles in women with preeclampsia-an exploratory study
Abstract
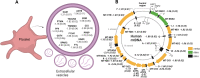
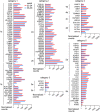
Background: Circulating extracellular vesicles (EVs) are increased in preeclampsia (PE) and are associated with severity and progression. We examined in this exploratory cohort study if the mRNAs and long noncoding RNAs (lncRNAs) in plasma-derived EVs were dysregulated in PE compared to normal pregnancy and display different temporal patterns during gestation.
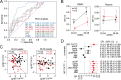
Methods: We isolated EVs from plasma at weeks 22-24 and 36-38 in women with and without PE (n=7 in each group) and performed RNA-seq, focusing on mRNAs and lncRNAs. We validated highly expressed mitochondrial and platelet-derived RNAs discovered from central pathways in 60 women with/without PE. We examined further one of the regulated RNAs, noncoding mitochondrially encoded tRNA alanine (MT-TA), in leukocytes and plasma to investigate its biomarker potential and association with clinical markers of PE.
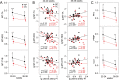
Results: We found abundant levels of platelet-derived and mitochondrial RNAs in EVs. Expression of these RNAs were decreased and lncRNAs increased in EVs from PE compared to without PE. These findings were further validated by qPCR for mitochondrial RNAs MT-TA, MT-ND2, MT-CYB and platelet-derived RNAs PPBP, PF4, CLU in EVs. Decreased expression of mitochondrial tRNA MT-TA in leukocytes at 22-24 weeks was strongly associated with the subsequent development of PE.
Conclusions: Platelet-derived and mitochondrial RNA were highly expressed in plasma EVs and were decreased in EVs isolated from women with PE compared to without PE. LncRNAs were mostly increased in PE. The MT-TA in leukocytes may be a useful biomarker for prediction and/or early detection of PE.
Keywords: Extracellular vesicles; Preeclampsia; lncRNA.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Exosomal small RNA profiling in first-trimester maternal blood explores early molecular pathways of preterm preeclampsia.Front Immunol. 2024 Feb 22;15:1321191. doi: 10.3389/fimmu.2024.1321191. eCollection 2024. Front Immunol. 2024. PMID: 38455065 Free PMC article.
-
Differential 5'-tRNA Fragment Expression in Circulating Preeclampsia Syncytiotrophoblast Vesicles Drives Macrophage Inflammation.Hypertension. 2024 Apr;81(4):876-886. doi: 10.1161/HYPERTENSIONAHA.123.22292. Epub 2024 Feb 16. Hypertension. 2024. PMID: 38362745 Free PMC article.
-
Small extracellular vesicles-transported lncRNA TDRKH-AS1 derived from AOPPs-treated trophoblasts initiates endothelial cells pyroptosis through PDIA4/DDIT4 axis in preeclampsia.J Transl Med. 2023 Jul 24;21(1):496. doi: 10.1186/s12967-023-04346-6. J Transl Med. 2023. PMID: 37488572 Free PMC article.
-
Overview of extracellular vesicles in the pathogenesis of preeclampsia†.Biol Reprod. 2021 Jul 2;105(1):32-39. doi: 10.1093/biolre/ioab060. Biol Reprod. 2021. PMID: 33778844 Review.
-
Extracellular Vesicles and Preeclampsia: Current Knowledge and Future Research Directions.Subcell Biochem. 2021;97:455-482. doi: 10.1007/978-3-030-67171-6_18. Subcell Biochem. 2021. PMID: 33779928 Review.
Cited by
-
The long non-coding RPPH1 is decreased in leukocytes and increased in plasma from women developing pre-eclampsia†.Biol Reprod. 2024 Aug 15;111(2):427-435. doi: 10.1093/biolre/ioae069. Biol Reprod. 2024. PMID: 38685609 Free PMC article.
-
Platelets in preeclampsia: an observational study of indices associated with aspirin nonresponsiveness, activation and transcriptional landscape.BMC Med. 2025 Jun 9;23(1):346. doi: 10.1186/s12916-025-04132-9. BMC Med. 2025. PMID: 40490753 Free PMC article.
-
LINC01410 accelerates the invasion of trophoblast cells by modulating METTL3/Fas.Mol Biol Rep. 2024 Aug 8;51(1):895. doi: 10.1007/s11033-024-09834-6. Mol Biol Rep. 2024. PMID: 39115693 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

