A rare non-canonical splice site in Trema orientalis SYMRK does not affect its dual symbiotic functioning in endomycorrhiza and rhizobium nodulation
- PMID: 37996841
- PMCID: PMC10668435
- DOI: 10.1186/s12870-023-04594-0
A rare non-canonical splice site in Trema orientalis SYMRK does not affect its dual symbiotic functioning in endomycorrhiza and rhizobium nodulation
Abstract
Background: Nitrogen-fixing nodules occur in ten related taxonomic lineages interspersed with lineages of non-nodulating plant species. Nodules result from an endosymbiosis between plants and diazotrophic bacteria; rhizobia in the case of legumes and Parasponia and Frankia in the case of actinorhizal species. Nodulating plants share a conserved set of symbiosis genes, whereas related non-nodulating sister species show pseudogenization of several key nodulation-specific genes. Signalling and cellular mechanisms critical for nodulation have been co-opted from the more ancient plant-fungal arbuscular endomycorrhizal symbiosis. Studies in legumes and actinorhizal plants uncovered a key component in symbiotic signalling, the LRR-type SYMBIOSIS RECEPTOR KINASE (SYMRK). SYMRK is essential for nodulation and arbuscular endomycorrhizal symbiosis. To our surprise, however, despite its arbuscular endomycorrhizal symbiosis capacities, we observed a seemingly critical mutation in a donor splice site in the SYMRK gene of Trema orientalis, the non-nodulating sister species of Parasponia. This led us to investigate the symbiotic functioning of SYMRK in the Trema-Parasponia lineage and to address the question of to what extent a single nucleotide polymorphism in a donor splice site affects the symbiotic functioning of SYMRK.
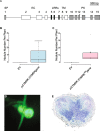
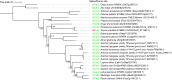
Results: We show that SYMRK is essential for nodulation and endomycorrhization in Parasponia andersonii. Subsequently, it is revealed that the 5'-intron donor splice site of SYMRK intron 12 is variable and, in most dicotyledon species, doesn't contain the canonical dinucleotide 'GT' signature but the much less common motif 'GC'. Strikingly, in T. orientalis, this motif is converted into a rare non-canonical 5'-intron donor splice site 'GA'. This SYMRK allele, however, is fully functional and spreads in the T. orientalis population of Malaysian Borneo. A further investigation into the occurrence of the non-canonical GA-AG splice sites confirmed that these are extremely rare.
Conclusion: SYMRK functioning is highly conserved in legumes, actinorhizal plants, and Parasponia. The gene possesses a non-common 5'-intron GC donor splice site in intron 12, which is converted into a GA in T. orientalis accessions of Malaysian Borneo. The discovery of this functional GA-AG splice site in SYMRK highlights a gap in our understanding of splice donor sites.
Keywords: Arbuscular mycorrhizal symbiosis; Common symbiosis signalling pathway; LRR-type transmembrane receptor kinase; Mutualistic endosymbiosis; Nitrogen-fixing nodulation symbiosis; Non-canonical splice site; Parasponia andersonii; Plant evolution; SYMRK; Trema orientalis.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Pseudogenization of the rhizobium-responsive EXOPOLYSACCHARIDE RECEPTOR in Parasponia is a rare event in nodulating plants.BMC Plant Biol. 2022 Apr 30;22(1):225. doi: 10.1186/s12870-022-03606-9. BMC Plant Biol. 2022. PMID: 35490231 Free PMC article.
-
Comparative genomics of the nonlegume Parasponia reveals insights into evolution of nitrogen-fixing rhizobium symbioses.Proc Natl Acad Sci U S A. 2018 May 15;115(20):E4700-E4709. doi: 10.1073/pnas.1721395115. Epub 2018 May 1. Proc Natl Acad Sci U S A. 2018. PMID: 29717040 Free PMC article.
-
Duplication of Symbiotic Lysin Motif Receptors Predates the Evolution of Nitrogen-Fixing Nodule Symbiosis.Plant Physiol. 2020 Oct;184(2):1004-1023. doi: 10.1104/pp.19.01420. Epub 2020 Jul 15. Plant Physiol. 2020. PMID: 32669419 Free PMC article.
-
Evolution of NIN and NIN-like Genes in Relation to Nodule Symbiosis.Genes (Basel). 2020 Jul 11;11(7):777. doi: 10.3390/genes11070777. Genes (Basel). 2020. PMID: 32664480 Free PMC article. Review.
-
Recent advances in actinorhizal symbiosis signaling.Plant Mol Biol. 2016 Apr;90(6):613-22. doi: 10.1007/s11103-016-0450-2. Epub 2016 Feb 12. Plant Mol Biol. 2016. PMID: 26873697 Review.
Cited by
-
Ectopic expression of the GRAS-type transcriptional regulator NSP2 in Parasponia triggers contrasting effects on symbioses.Front Plant Sci. 2024 Oct 30;15:1468812. doi: 10.3389/fpls.2024.1468812. eCollection 2024. Front Plant Sci. 2024. PMID: 39539299 Free PMC article.
References
-
- Duc G, Trouvelot A, Gianinazzi-Pearson V, Gianinazzi S. First report of non-mycorrhizal plant mutants (Myc−) obtained in pea (Pisum sativum L.) and fababean (Vicia faba L.) Plant Sci. 1989;60:215–22. doi: 10.1016/0168-9452(89)90169-6. - DOI
MeSH terms
Substances
Grants and funding
- 10598/Ministry of Education, King Faisal University, Saudi Arabia
- 8245-ID/Ministry of Research, Technology, and Higher Education of the Republic of Indonesia
- OPP1172165/ENSA project funded by the Bill & Melinda Gates Foundation to the University of Cambridge
- OPP1172165/ENSA project funded by the Bill & Melinda Gates Foundation to the University of Cambridge
- OPP1172165/ENSA project funded by the Bill & Melinda Gates Foundation to the University of Cambridge
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

