Parathyroid hormone stimulates bone regeneration in an atrophic non-union model in aged mice
- PMID: 37996876
- PMCID: PMC10668449
- DOI: 10.1186/s12967-023-04661-y
Parathyroid hormone stimulates bone regeneration in an atrophic non-union model in aged mice
Erratum in
-
Correction to: Parathyroid hormone stimulates bone regeneration in an atrophic non-union model in aged mice.J Transl Med. 2024 Sep 9;22(1):828. doi: 10.1186/s12967-024-05594-w. J Transl Med. 2024. PMID: 39252093 Free PMC article. No abstract available.
Abstract
Background: Non-union formation still represents a major burden in trauma and orthopedic surgery. Moreover, aged patients are at an increased risk for bone healing failure. Parathyroid hormone (PTH) has been shown to accelerate fracture healing in young adult animals. However, there is no information whether PTH also stimulates bone regeneration in atrophic non-unions in the aged. Therefore, the aim of the present study was to analyze the effect of PTH on bone regeneration in an atrophic non-union model in aged CD-1 mice.
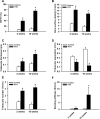
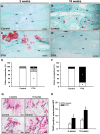
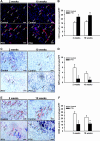
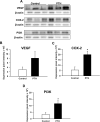
Methods: After creation of a 1.8 mm segmental defect, mice femora were stabilized by pin-clip fixation. The animals were treated daily with either 200 µg/kg body weight PTH 1-34 (n = 17) or saline (control; n = 17) subcutaneously. Bone regeneration was analyzed by means of X-ray, biomechanics, micro-computed tomography (µCT) imaging as well as histological, immunohistochemical and Western blot analyses.
Results: In PTH-treated animals bone formation was markedly improved when compared to controls. This was associated with an increased bending stiffness as well as a higher number of tartrate-resistant acid phosphatase (TRAP)-positive osteoclasts and CD31-positive microvessels within the callus tissue. Furthermore, PTH-treated aged animals showed a decreased inflammatory response, characterized by a lower number of MPO-positive granulocytes and CD68-positive macrophages within the bone defects when compared to controls. Additional Western blot analyses demonstrated a significantly higher expression of cyclooxygenase (COX)-2 and phosphoinositide 3-kinase (PI3K) in PTH-treated mice.
Conclusion: Taken together, these findings indicate that PTH is an effective pharmacological compound for the treatment of non-union formation in aged animals.
Keywords: Aging; Angiogenesis; Bone regeneration; Fracture healing; Inflammation; Mice; Non-union; Parathyroid hormone; Segmental defect.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Sildenafil, a phosphodiesterase-5 inhibitor, stimulates angiogenesis and bone regeneration in an atrophic non-union model in mice.J Transl Med. 2023 Sep 8;21(1):607. doi: 10.1186/s12967-023-04441-8. J Transl Med. 2023. PMID: 37684656 Free PMC article.
-
Cilostazol promotes blood vessel formation and bone regeneration in a murine non-union model.Biomed Pharmacother. 2023 Dec;168:115697. doi: 10.1016/j.biopha.2023.115697. Epub 2023 Oct 19. Biomed Pharmacother. 2023. PMID: 37864892
-
Cilostazol Stimulates Angiogenesis and Accelerates Fracture Healing in Aged Male and Female Mice by Increasing the Expression of PI3K and RUNX2.Int J Mol Sci. 2024 Jan 6;25(2):755. doi: 10.3390/ijms25020755. Int J Mol Sci. 2024. PMID: 38255829 Free PMC article.
-
Parathyroid hormone for bone regeneration.J Orthop Res. 2018 Oct;36(10):2586-2594. doi: 10.1002/jor.24075. Epub 2018 Jul 23. J Orthop Res. 2018. PMID: 29926970 Review.
-
Parathyroid hormone and bone healing.Calcif Tissue Int. 2010 Jul;87(1):1-13. doi: 10.1007/s00223-010-9360-5. Epub 2010 Apr 29. Calcif Tissue Int. 2010. PMID: 20428858 Review.
Cited by
-
Cortisol stress response after musculoskeletal surgery: a narrative review.EFORT Open Rev. 2025 Apr 1;10(4):186-192. doi: 10.1530/EOR-2024-0126. EFORT Open Rev. 2025. PMID: 40167425 Free PMC article. Review.
-
Injective hydrogel loaded with liposomes-encapsulated MY-1 promotes wound healing and increases tensile strength by accelerating fibroblast migration via the PI3K/AKT-Rac1 signaling pathway.J Nanobiotechnology. 2024 Jul 5;22(1):396. doi: 10.1186/s12951-024-02666-3. J Nanobiotechnology. 2024. PMID: 38965546 Free PMC article.
-
Fortified Withaferin A accelerates the transition from fibrovascular to bone remodeling phase during endochondral bone formation to promote ossification.Front Endocrinol (Lausanne). 2025 Apr 25;16:1540237. doi: 10.3389/fendo.2025.1540237. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40352455 Free PMC article.
-
Correction to: Parathyroid hormone stimulates bone regeneration in an atrophic non-union model in aged mice.J Transl Med. 2024 Sep 9;22(1):828. doi: 10.1186/s12967-024-05594-w. J Transl Med. 2024. PMID: 39252093 Free PMC article. No abstract available.
-
Novel FRMD6::PTH chimera in tumorous bone lesion carrying a t(4;11;14;12)(q35;p15;q22;q13).Pathol Oncol Res. 2025 Jun 26;31:1612096. doi: 10.3389/pore.2025.1612096. eCollection 2025. Pathol Oncol Res. 2025. PMID: 40641561 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

