Pathologically catalyzed physical coating restores the intestinal barrier for inflammatory bowel disease therapy
- PMID: 37996883
- PMCID: PMC10668504
- DOI: 10.1186/s12951-023-02227-0
Pathologically catalyzed physical coating restores the intestinal barrier for inflammatory bowel disease therapy
Abstract
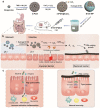
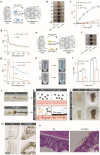
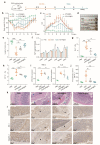
Intestinal epithelia impairment of inflammatory bowel disease (IBD) leads to the leakage of bacteria and antigens and the consequent persistent immune imbalance. Restoring the epithelial barrier is a promising therapeutic target but lacks effective and safe clinical interventions. By identifying the catalase (CAT) presence in the IBD pathological environment, we herein develop a CAT-catalyzed pathologically coating on the damaged epithelial barrier to inhibit intestinal leakage for IBD therapy. With the codelivery of CaO2 (a CAT substrate) and dopamine, the nanosystem can enable CAT-catalyzed oxygen (O2) production and in-situ polymerization of dopamine and then yield a thin and integrative polydopamine (PDA) coating on the intestinal barrier due to the highly adhesive property of PDA. In vivo study demonstrates that PDA coating provides not only a protective barrier by restricting intestinal leakage but also a favorable anti-inflammation effect. Beyond drug management, this work provides a physical repair strategy via catalyzed coating for IBD therapy.
Keywords: Inflammatory bowel Disease; Intestinal barrier; Pathological catalysis; Polydopamine coating.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Catalase catalyzed tannic acid-Fe3+ network coating: A theranostic strategy for intestinal barrier restoration.Int J Biol Macromol. 2024 Aug;274(Pt 2):133304. doi: 10.1016/j.ijbiomac.2024.133304. Epub 2024 Jun 24. Int J Biol Macromol. 2024. PMID: 38925189
-
GelNB molecular coating as a biophysical barrier to isolate intestinal irritating metabolites and regulate intestinal microbial homeostasis in the treatment of inflammatory bowel disease.Bioact Mater. 2022 Apr 21;19:251-267. doi: 10.1016/j.bioactmat.2022.04.001. eCollection 2023 Jan. Bioact Mater. 2022. PMID: 35510173 Free PMC article.
-
Broad-Spectrum ROS/RNS Scavenging Catalase-Loaded Microreactors for Effective Oral Treatment of Inflammatory Bowel Diseases.Small. 2025 Jun;21(23):e2501341. doi: 10.1002/smll.202501341. Epub 2025 Apr 22. Small. 2025. PMID: 40263925
-
Interaction Between Commensal Bacteria, Immune Response and the Intestinal Barrier in Inflammatory Bowel Disease.Front Immunol. 2021 Nov 11;12:761981. doi: 10.3389/fimmu.2021.761981. eCollection 2021. Front Immunol. 2021. PMID: 34858414 Free PMC article. Review.
-
Intestinal enteroids/organoids: A novel platform for drug discovery in inflammatory bowel diseases.World J Gastroenterol. 2019 Aug 14;25(30):4125-4147. doi: 10.3748/wjg.v25.i30.4125. World J Gastroenterol. 2019. PMID: 31435168 Free PMC article. Review.
Cited by
-
Restore Intestinal Barrier Integrity: An Approach for Inflammatory Bowel Disease Therapy.J Inflamm Res. 2024 Aug 14;17:5389-5413. doi: 10.2147/JIR.S470520. eCollection 2024. J Inflamm Res. 2024. PMID: 39161679 Free PMC article. Review.
References
-
- Parikh K, Antanaviciute A, Fawkner-Corbett D, Jagielowicz M, Aulicino A, Lagerholm C, Davis S, Kinchen J, Chen HH, Alham NK, Ashley N, Johnson E, Hublitz P, Bao L, Lukomska J, Andev RS, Bjorklund E, Kessler BM, Fischer R, Goldin R, Koohy H, Simmons A. Colonic epithelial cell diversity in health and inflammatory bowel Disease. Nature. 2019;567(7746):49–55. doi: 10.1038/s41586-019-0992-y. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- 2021YFC2400600/National Key Research and Development Program of China
- 81925035/National Nature Science Foundation of China
- 81925035/National Nature Science Foundation of China
- 2022XD052/Talent Program of Shanghai Municipal Health Commission
- 2021ZD0202003/Sci-Tech Innovation 2030-Major Project of Brain science and brain-inspired intelligence technology
LinkOut - more resources
Full Text Sources
Miscellaneous

