Prenylated chromones and flavonoids isolated from the roots of Flemingia macrophylla and their anti-lung cancer activity
- PMID: 37996917
- PMCID: PMC10668522
- DOI: 10.1186/s13020-023-00860-3
Prenylated chromones and flavonoids isolated from the roots of Flemingia macrophylla and their anti-lung cancer activity
Abstract
Background: The successful launch of icaritin, a therapeutic drug for liver cancer derived from Epimedium brevicornu, has provided new impetus for the development of prenylated flavonoids in the field of oncology. Flemingia macrophylla is reported to contain characteristic prenylated flavonoids which can regulate the p53 protein. We aimed to isolate these constituents and conduct activity evaluation, structure-activity relationship, and mechanism studies to provide candidate compounds for antitumor drug development.
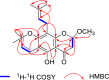
Methods: In this study, chromatographic techniques combined with spectroscopic methods were used to separate, purify, and identify the constituents of Flemingia macrophylla methanol extract. The cytotoxic activity of the constituents was evaluated using an MTT assay with A549 and H1975 cells as the model. The binding mechanism between the compounds and the p53 protein was investigated with molecular docking and validated with cellular thermal shift assay (CETSA). Western blotting (WB) was employed to detect the expression of p53 protein and apoptosis-related proteins in cells.
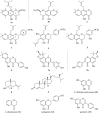
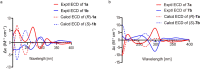
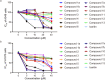
Results: Chiral HPLC separation of racemates 1 and 7 provided two pairs of undescribed enantiomers (1a/1b and 7a/7b), along with eight known compounds (2 - 9) isolated from Flemingia macrophylla roots. Their structures were elucidated by spectroscopic analysis, and the absolute configurations of the enantiomers were determined from experimental and calculated electronic circular dichroism data. Compounds 1 - 7, and the non-prenyl analogues 10 - 13, were evaluated for cytotoxic activity against the human lung cancer A549 and H1975 cell line. Compounds 5 - 7 displayed better cytotoxicity than the positive control icaritin in A549 and H1975, with IC50 values ranging from 4.50 to 19.83 μmol·L-1 and < 5 μmol·L-1, respectively. The structure-activity relationships of the chromone or flavonoid analogues against A549 cells were discussed. Molecular docking results demonstrated that compound 7a has strong interaction with p53 and WB indicated that 7a induced apoptosis by increasing the p53 protein, decreasing the anti-apoptotic protein Bcl-2, and activating the caspase family in A549 cells. These results suggest that prenylated flavonoids are potential p53 protein activators.
Conclusion: This study demonstrates that Flemingia macrophylla is rich in prenylated flavonoid constituents, among which compounds 5 and 7 exhibited significant cytotoxic activity against A549 cells and served as reference candidates for the design and development of prenylated compounds as antitumor therapeutic drugs.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
A flavonoid isolated from Streptomyces sp. (ERINLG-4) induces apoptosis in human lung cancer A549 cells through p53 and cytochrome c release caspase dependant pathway.Chem Biol Interact. 2014 Dec 5;224:24-35. doi: 10.1016/j.cbi.2014.09.019. Epub 2014 Oct 5. Chem Biol Interact. 2014. PMID: 25289772
-
Prenylated flavonoids from Ficus hirta induces HeLa cells apoptosis via MAPK and AKT signaling pathways.Bioorg Med Chem Lett. 2021 Apr 15;38:127859. doi: 10.1016/j.bmcl.2021.127859. Epub 2021 Feb 18. Bioorg Med Chem Lett. 2021. PMID: 33609662
-
Six pairs of enantiomeric prenylated flavonoids with cytotoxic activities from Epimedium sagittatum Maxim.Nat Prod Bioprospect. 2025 May 13;15(1):31. doi: 10.1007/s13659-025-00510-1. Nat Prod Bioprospect. 2025. PMID: 40358625 Free PMC article.
-
A comprehensive review: Biological activity, modification and synthetic methodologies of prenylated flavonoids.Phytochemistry. 2021 Nov;191:112895. doi: 10.1016/j.phytochem.2021.112895. Epub 2021 Aug 14. Phytochemistry. 2021. PMID: 34403885 Review.
-
Prenylated Flavonoids of the Moraceae Family: A Comprehensive Review of Their Biological Activities.Plants (Basel). 2024 Apr 27;13(9):1211. doi: 10.3390/plants13091211. Plants (Basel). 2024. PMID: 38732426 Free PMC article. Review.
Cited by
-
High-quality assembly of the chromosomal genome for Flemingia macrophylla reveals genomic structural characteristics.BMC Genomics. 2025 May 26;26(1):535. doi: 10.1186/s12864-025-11705-8. BMC Genomics. 2025. PMID: 40419955 Free PMC article.
-
Integrated bioinformatics and network pharmacology to identify and validate macrophage polarization related hub genes in the treatment of osteoarthritis with Astragalus membranaceus.J Orthop Surg Res. 2025 May 30;20(1):543. doi: 10.1186/s13018-025-05799-9. J Orthop Surg Res. 2025. PMID: 40442788 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

