Characterizing lysine acetylation of glucokinase
- PMID: 37996965
- PMCID: PMC10731539
- DOI: 10.1002/pro.4845
Characterizing lysine acetylation of glucokinase
Abstract
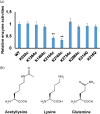
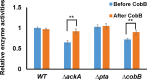
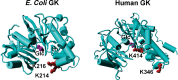
Glucokinase (GK) catalyzes the phosphorylation of glucose to form glucose-6-phosphate as the substrate of glycolysis for energy production. Acetylation of lysine residues in Escherichia coli GK has been identified at multiple sites by a series of proteomic studies, but the impact of acetylation on GK functions remains largely unknown. In this study, we applied the genetic code expansion strategy to produce site-specifically acetylated GK variants which naturally exist in cells. Enzyme assays and kinetic analyses showed that lysine acetylation decreases the GK activity, mostly resulting from acetylation of K214 and K216 at the entrance of the active site, which impairs the binding of substrates. We also compared results obtained from the glutamine substitution method and the genetic acetyllysine incorporation approach, showing that glutamine substitution is not always effective for mimicking acetylated lysine. Further genetic studies as well as in vitro acetylation and deacetylation assays were performed to determine acetylation and deacetylation mechanisms, which showed that E. coli GK could be acetylated by acetyl-phosphate without enzymes and deacetylated by CobB deacetylase.
Keywords: deacetylase; genetic code expansion; glucokinase; glycolysis; lysine acetylation.
© 2023 The Protein Society.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

