Corticotropin-releasing factor-dopamine interactions in male and female macaque: Beyond the classic VTA
- PMID: 37996987
- PMCID: PMC10842953
- DOI: 10.1002/syn.22284
Corticotropin-releasing factor-dopamine interactions in male and female macaque: Beyond the classic VTA
Abstract
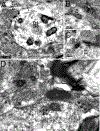
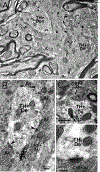
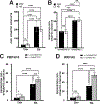
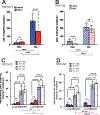
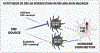
Dopamine (DA) is involved in stress and stress-related illnesses, including many psychiatric disorders. Corticotropin-releasing factor (CRF) plays a role in stress responses and targets the ventral midbrain DA system, which is composed of DA and non-DA cells, and divided into specific subregions. Although CRF inputs to the midline A10 nuclei ("classic VTA") are known, in monkeys, CRF-containing terminals are also highly enriched in the expanded A10 parabrachial pigmented nucleus (PBP) and in the A8 retrorubral field subregions. We characterized CRF-labeled synaptic terminals on DA (tyrosine hydroxylase, TH+) and non-DA (TH-) cell types in the PBP and A8 regions using immunoreactive electron microscopy (EM) in male and female macaques. CRF labeling was present mostly in axon terminals, which mainly contacted TH-negative dendrites in both subregions. Most CRF-positive terminals had symmetric profiles. In both PBP and A8, CRF symmetric (putative inhibitory) synapses onto TH-negative dendrites were significantly greater than asymmetric (putative excitatory) profiles. This overall pattern was similar in males and females, despite shifts in the size of these effects between regions depending on sex. Because stress and gonadal hormone shifts can influence CRF expression, we also did hormonal assays over a 6-month time period and found little variability in basal cortisol across similarly housed animals at the same age. Together our findings suggest that at baseline, CRF-positive synaptic terminals in the primate PBP and A8 are poised to regulate DA indirectly through synaptic contacts onto non-DA neurons.
Keywords: A8); corticotropin; dopamine; electron microscopy; nonhuman primate; parabrachial nucleus (PBP); retrorubral field (RRF; ventral tegmental area (VTA).
© 2023 Wiley Periodicals LLC.
Conflict of interest statement
Competing Interests.
The authors have no financial or non-financial interests to disclose.
Figures



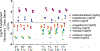
References
-
- Arsenault MY, Parent A, Seguela P, Descarries L (1988) Distribution and morphological characteristics of dopamine-immunoreactive neurons in the midbrain of the squirrel monkey (Saimiri sciureus). J Comp Neurol 267:489–506 - PubMed
-
- Bangasser DA, Reyes BA, Piel D, Garachh V, Zhang XY, Plona ZM, Van Bockstaele EJ, Beck SG, Valentino RJ (2013) Increased vulnerability of the brain norepinephrine system of females to corticotropin-releasing factor overexpression. Molecular psychiatry 18 (2):166–173. doi: 10.1038/mp.2012.24 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
