Vericiguat, a novel sGC stimulator: Mechanism of action, clinical, and translational science
- PMID: 37997225
- PMCID: PMC10719466
- DOI: 10.1111/cts.13677
Vericiguat, a novel sGC stimulator: Mechanism of action, clinical, and translational science
Abstract
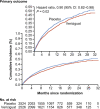
Vericiguat, a novel soluble guanylate cyclase (sGC) stimulator, is approved for the treatment of heart failure (HF) with reduced ejection fraction (HFrEF). Decreased nitric oxide (NO) availability, sGC desensitization to NO, sGC deficiency, and reduced cyclic guanosine monophosphate (cGMP) signaling are potential contributing factors for HF disease progression. Vericiguat works via stimulation of sGC in the critical NO-sGC-cGMP pathway. Vericiguat is primarily metabolized by glucuronidation via uridine diphosphate-glucuronosyltransferase (UGT) isoforms UGT1A1 and UGT1A9. Urinary excretion and renal clearance of vericiguat are low. No intrinsic factor had a clinically relevant effect on vericiguat exposure. Vericiguat has low drug-drug interaction potential with no clinically relevant pharmacokinetic or pharmacodynamic interactions observed with warfarin, digoxin, aspirin, or sacubitril/valsartan. The global phase III study VICTORIA included patients with HFrEF who had a recent HF hospitalization or intravenous diuretic treatment for HF. Treatment with vericiguat on top of standard of care resulted in a 10% relative reduction in the primary composite outcome of death from cardiovascular causes or first hospitalization for HF. Vericiguat was well-tolerated with low incidence of symptomatic hypotension and syncope compared to placebo. Given its positive benefit-risk profile, vericiguat is an important option for high-risk patients with HFrEF who are already on guideline-directed medical therapy and had recent worsening of HF. Future efforts to develop additional effective therapies are needed to further reduce morbidity and mortality in patients with HF.
© 2023 Merck Sharp & Dohme LLC. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
M.E.T., S.A., R.O.B., and F.G. are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and may own stock and/or stock options in Merck & Co., Inc., Rahway, NJ, USA.
Figures

References
-
- Food and Drug Administration . Verquvo™ (vericiguat) tablets. Highlights of prescribing information. Accessed May 11, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214377s000lbl.pdf
-
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789‐1858. doi: 10.1016/S0140-6736(18)32279-7 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

