Thermally Stable Terbium(II) and Dysprosium(II) Bis-amidinate Complexes
- PMID: 37997752
- PMCID: PMC10755703
- DOI: 10.1021/jacs.3c07978
Thermally Stable Terbium(II) and Dysprosium(II) Bis-amidinate Complexes
Abstract
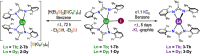
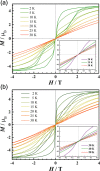
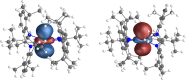
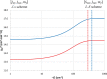
The thermostable four-coordinate divalent lanthanide (Ln) bis-amidinate complexes [Ln(Piso)2] (Ln = Tb, Dy; Piso = {(NDipp)2CtBu}, Dipp = C6H3iPr2-2,6) were prepared by the reduction of parent five-coordinate Ln(III) precursors [Ln(Piso)2I] (Ln = Tb, Dy) with KC8; halide abstraction of [Ln(Piso)2I] with [H(SiEt3)2][B(C6F5)] gave the respective Ln(III) complexes [Ln(Piso)2][B(C6F5)]. All complexes were characterized by X-ray diffraction, ICP-MS, elemental analysis, SQUID magnetometry, UV-vis-NIR, ATR-IR, NMR, and EPR spectroscopy and ab initio CASSCF-SO calculations. These data consistently show that [Ln(Piso)2] formally exhibit Ln(II) centers with 4fn5dz21 (Ln = Tb, n = 8; Dy, n = 9) valence electron configurations. We show that simple assignments of the f-d coupling to either L-S or J-s schemes are an oversimplification, especially in the presence of significant crystal field splitting. The coordination geometry of [Ln(Piso)2] is intermediate between square planar and tetrahedral. Projecting from the quaternary carbon atoms of the CN2 ligand backbones shows near-linear C···Ln···C arrangements. This results in strong axial ligand fields to give effective energy barriers to magnetic reversal of 1920(91) K for the Tb(II) analogue and 1964(48) K for Dy(II), the highest values observed for mononuclear Ln(II) single-molecule magnets, eclipsing 1738 K for [Tb(C5iPr5)2]. We tentatively attribute the fast zero-field magnetic relaxation for these complexes at low temperatures to transverse fields, resulting in considerable mixing of mJ states.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- The Lanthanides and Actinides; Liddle S. T.; Mills D. P.; Natrajan L. S., Eds.; World Scientific Publishing Europe Ltd.: Singapore, 2022.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

