Proton Microbeam Targeted Irradiation of the Gonad Primordium Region Induces Developmental Alterations Associated with Heat Shock Responses and Cuticle Defense in Caenorhabditis elegans
- PMID: 37997971
- PMCID: PMC10669138
- DOI: 10.3390/biology12111372
Proton Microbeam Targeted Irradiation of the Gonad Primordium Region Induces Developmental Alterations Associated with Heat Shock Responses and Cuticle Defense in Caenorhabditis elegans
Abstract
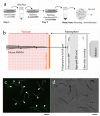
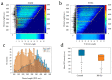
We describe a methodology to manipulate Caenorhabditis elegans (C. elegans) and irradiate the stem progenitor gonad region using three MeV protons at a specific developmental stage (L1). The consequences of the targeted irradiation were first investigated by considering the organogenesis of the vulva and gonad, two well-defined and characterized developmental systems in C. elegans. In addition, we adapted high-throughput analysis protocols, using cell-sorting assays (COPAS) and whole transcriptome analysis, to the limited number of worms (>300) imposed by the selective irradiation approach. Here, the presented status report validated protocols to (i) deliver a controlled dose in specific regions of the worms; (ii) immobilize synchronized worm populations (>300); (iii) specifically target dedicated cells; (iv) study the radiation-induced developmental alterations and gene induction involved in cellular stress (heat shock protein) and cuticle injury responses that were found.
Keywords: COPAS; Caenorhabditis elegans; gonadal–vulval development; microbeam; nanopore sequencing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources

