Role of Stress on Driving the Intestinal Paracellular Permeability
- PMID: 37998758
- PMCID: PMC10670774
- DOI: 10.3390/cimb45110581
Role of Stress on Driving the Intestinal Paracellular Permeability
Abstract
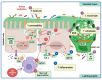
The gut epithelium is a polarized monolayer that exhibits apical and basolateral membrane surfaces. Monolayer cell components are joined side by side via protein complexes known as tight junction proteins (TJPs), expressed at the most apical extreme of the basolateral membrane. The gut epithelium is a physical barrier that determinates intestinal permeability, referred to as the measurement of the transit of molecules from the intestinal lumen to the bloodstream or, conversely, from the blood to the gut lumen. TJPs play a role in the control of intestinal permeability that can be disrupted by stress through signal pathways triggered by the ligation of receptors with stress hormones like glucocorticoids. Preclinical studies conducted under in vitro and/or in vivo conditions have addressed underlying mechanisms that account for the impact of stress on gut permeability. These mechanisms may provide insights for novel therapeutic interventions in diseases in which stress is a risk factor, like irritable bowel syndrome. The focus of this study was to review, in an integrative context, the neuroendocrine effects of stress, with special emphasis on TJPs along with intestinal permeability.
Keywords: claudins; corticosterone; gut epithelium; occludin; paracellular permeability; stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Intestinal Alkaline Phosphatase Prevents Sulfate Reducing Bacteria-Induced Increased Tight Junction Permeability by Inhibiting Snail Pathway.Front Cell Infect Microbiol. 2022 May 26;12:882498. doi: 10.3389/fcimb.2022.882498. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35694541 Free PMC article.
-
Stimulation of apical and basolateral VEGF-A and VEGF-C secretion by oxidative stress in polarized retinal pigment epithelial cells.Mol Vis. 2006 Dec 22;12:1649-59. Mol Vis. 2006. PMID: 17200665
-
Enhanced gastrointestinal passive paracellular permeability contributes to the obesity-associated hyperoxaluria.Am J Physiol Gastrointest Liver Physiol. 2019 Jan 1;316(1):G1-G14. doi: 10.1152/ajpgi.00266.2018. Epub 2018 Oct 11. Am J Physiol Gastrointest Liver Physiol. 2019. PMID: 30307745 Free PMC article.
-
Enteric Pathogens and Their Toxin-Induced Disruption of the Intestinal Barrier through Alteration of Tight Junctions in Chickens.Toxins (Basel). 2017 Feb 10;9(2):60. doi: 10.3390/toxins9020060. Toxins (Basel). 2017. PMID: 28208612 Free PMC article. Review.
-
Emerging roles of transmembrane-type tight junction proteins in cancers.Pathol Int. 2023 Aug;73(8):331-340. doi: 10.1111/pin.13349. Epub 2023 Jul 14. Pathol Int. 2023. PMID: 37449777 Review.
Cited by
-
Looking Inside of the Intestinal Permeability Regulation by Protein-Derivatives from Bovine Milk.Mol Nutr Food Res. 2024 Nov;68(22):e2400384. doi: 10.1002/mnfr.202400384. Epub 2024 Nov 12. Mol Nutr Food Res. 2024. PMID: 39530631 Free PMC article. Review.
-
Psychobiotics and the Microbiota-Gut-Brain Axis: Where Do We Go from Here?Microorganisms. 2024 Mar 22;12(4):634. doi: 10.3390/microorganisms12040634. Microorganisms. 2024. PMID: 38674579 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

