The Early Endocytosis Gene PAL1 Contributes to Stress Tolerance and Hyphal Formation in Candida albicans
- PMID: 37998902
- PMCID: PMC10672141
- DOI: 10.3390/jof9111097
The Early Endocytosis Gene PAL1 Contributes to Stress Tolerance and Hyphal Formation in Candida albicans
Abstract
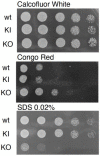
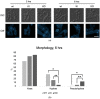
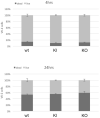
The endocytic and secretory pathways of the fungal pathogen Candida albicans are fundamental to various key cellular processes such as cell growth, cell wall integrity, protein secretion, hyphal formation, and pathogenesis. Our previous studies focused on several candidate genes involved in early endocytosis, including ENT2 and END3, that play crucial roles in such processes. However, much remains to be discovered about other endocytosis-related genes and their contributions toward Candida albicans secretion and virulence. In this study, we examined the functions of the early endocytosis gene PAL1 using a reverse genetics approach based on CRISPR-Cas9-mediated gene deletion. Saccharomyces cerevisiae Pal1 is a protein in the early coat complex involved in clathrin-mediated endocytosis that is later internalized with the coat. The C. albicans pal1Δ/Δ null mutant demonstrated increased resistance to the antifungal agent caspofungin and the cell wall stressor Congo Red. In contrast, the null mutant was more sensitive to the antifungal drug fluconazole and low concentrations of SDS than the wild type (WT) and the re-integrant (KI). While pal1Δ/Δ can form hyphae and a biofilm, under some hyphal-inducing conditions, it was less able to demonstrate filamentous growth when compared to the WT and KI. The pal1Δ/Δ null mutant had no defect in clathrin-mediated endocytosis, and there were no changes in virulence-related processes compared to controls. Our results suggest that PAL1 has a role in susceptibility to antifungal agents, cell wall integrity, and membrane stability related to early endocytosis.
Keywords: Candida albicans; clathrin-mediated endocytosis; hyphal formation; stress tolerance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Wächtler B., Citiulo F., Jablonowski N., Förster S., Dalle F., Schaller M., Wilson D., Hube B. Candida albicans-Epithelial Interactions: Dissecting the Roles of Active Penetration, Induced Endocytosis and Host Factors on the Infection Process. PLoS ONE. 2012;7:e36952. doi: 10.1371/journal.pone.0036952. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

