Genomic Epidemiology Identifies Azole Resistance Due to TR34/L98H in European Aspergillus fumigatus Causing COVID-19-Associated Pulmonary Aspergillosis
- PMID: 37998909
- PMCID: PMC10672581
- DOI: 10.3390/jof9111104
Genomic Epidemiology Identifies Azole Resistance Due to TR34/L98H in European Aspergillus fumigatus Causing COVID-19-Associated Pulmonary Aspergillosis
Abstract
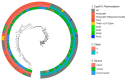
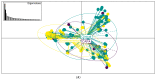
Aspergillus fumigatus has been found to coinfect patients with severe SARS-CoV-2 virus infection, leading to COVID-19-associated pulmonary aspergillosis (CAPA). The CAPA all-cause mortality rate is approximately 50% and may be complicated by azole resistance. Genomic epidemiology can help shed light on the genetics of A. fumigatus causing CAPA, including the prevalence of resistance-associated alleles. We present a population genomic analysis of 21 CAPA isolates from four European countries with these isolates compared against 240 non-CAPA A. fumigatus isolates from a wider population. Bioinformatic analysis and antifungal susceptibility testing were performed to quantify resistance and identify possible genetically encoded azole-resistant mechanisms. The phylogenetic analysis of the 21 CAPA isolates showed that they were representative of the wider A. fumigatus population with no obvious clustering. The prevalence of phenotypic azole resistance in CAPA was 14.3% (n = 3/21); all three CAPA isolates contained a known resistance-associated cyp51A polymorphism. The relatively high prevalence of azole resistance alleles that we document poses a probable threat to treatment success rates, warranting the enhanced surveillance of A. fumigatus genotypes in these patients. Furthermore, potential changes to antifungal first-line treatment guidelines may be needed to improve patient outcomes when CAPA is suspected.
Keywords: Aspergillus fumigatus; CAPA; COVID-19-associated pulmonary aspergillosis; azole-resistant Aspergillus fumigatus; coinfection; genetic epidemiology; genomic analysis.
Conflict of interest statement
M.C.F. and J.R. have received honoraria from Gilead Sciences for presentations. T.R. has received a research grant from Pfizer Healthcare Ireland outside of this work. A.F.T. has received a research grant from Gilead Sciences outside of this work. S.S. has received honoraria from Gilead Sciences and Pfizer for presentations.
Figures








References
-
- Ullmann A., Aguado J., Arikan-Akdagli S., Denning D., Groll A., Lagrou K., Lass-Flörl C., Munoz R.L.P., Verweij P., Warris A., et al. Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect. 2018;24:e1–e38. doi: 10.1016/j.cmi.2018.01.002. - DOI - PubMed
-
- Pakzad R., Malekifar P., Shateri Z., Zandi M., Rezayat S., Soleymani M., Karimi M., Ahmadi S., Shahbahrami R., Pakzad I., et al. Worldwide prevalence of microbial agents’ coinfection among COVID-19 patients: A comprehensive updated systematic review and meta-analysis. J. Clin. Lab. Anal. 2022;36:e24151. doi: 10.1002/jcla.24151. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

