An Evaluation of the OLM Pneum ID Real-Time Polymerase Chain Reaction to Aid in the Diagnosis of Pneumocystis Pneumonia
- PMID: 37998911
- PMCID: PMC10672265
- DOI: 10.3390/jof9111106
An Evaluation of the OLM Pneum ID Real-Time Polymerase Chain Reaction to Aid in the Diagnosis of Pneumocystis Pneumonia
Abstract
Background: The use of the PCR to aid in the diagnosis of Pneumocystis pneumonia (PcP) has demonstrated excellent clinical performance, as evidenced through various systematic reviews and meta-analyses, yet there are concerns over the interpretation of positive results due to the potential presence of Pneumocystis colonization of the airways. While this can be overcome by applying designated positivity thresholds to PCR testing, the shear number of assays described limits the development of a universal threshold. Commercial assays provide the opportunity to overcome this problem, provided satisfactory performance is determined through large-scale, multi-centre evaluations.
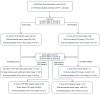
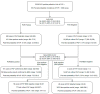
Methods: Retrospective case/control and consecutive cohort performance evaluations of the OLM PneumID real-time PCR assay were performed on DNA eluates from a range of samples sent from patients where "in-house" PCR had been performed as part of routine diagnostic testing. The clinical performance of the PneumID assay was determined before including it in a diagnostic algorithm to provide the probability of PcP (dependent on diagnostic evidence).
Results: After being used to test 317 patients (32 with PcP), the overall performance of the PneumID assay was found to be excellent (Sensitivity/Specificity: 96.9%/95.1%). False positivity could be removed by applying a threshold specific to sample type (<33.1 cycles for BAL fluid; <37.0 cycles for throat swabs), whereas considering any positive respiratory samples as significant generated 100% sensitivity, making absolute negativity sufficient to exclude PcP. Incorporating the PneumID assay into diagnostic algorithms alongside (1-3)-β-D-Glucan testing provided high probabilities of PcP (up to 85.2%) when both were positive and very low probabilities (<1%) when both were negative.
Conclusions: The OLM PneumID qPCR provides a commercial option for the accurate diagnosis of PcP, generating excellent sensitivity and specificity, particularly when testing respiratory specimens. The combination of PcP PCR with serum (1-3)-β-D-Glucan provides excellent clinical utility for diagnosing PcP.
Keywords: OLM PneumID; PcP PCR; Pneumocystis jirovecii; Pneumocystosis.
Conflict of interest statement
P.L.W.: Performed diagnostic evaluations and received meeting sponsorship from Associates of Cape Cod, Bruker, Dynamiker, and Launch Diagnostics; speaker’s fees, expert advice fees, and meeting sponsorship from Gilead; and speaker and expert advice fees from Pfizer and expert advice fees from F2G and Mundipharma. M.B.: Speaker’s fees and advisory board fees from Gilead, GlaxoSmithKline, Insmed, and Pfizer. J.S.P.: Received speaker’s fees from Pfizer. All other authors declare no conflicts of interest.
Figures
References
-
- Summah H., Zhu Y.G., Falagas M.E., Vouloumanou E.K., Qu J.M. Use of real-time polymerase chain reaction for the diagnosis of Pneumocystis pneumonia in immunocompromised patients: A meta-analysis. Chin. Med. J. 2013;126:1965–1973. - PubMed
-
- Senécal J., Smyth E., Del Corpo O., Hsu J.M., Amar-Zifkin A., Bergeron A., Cheng M.P., Butler-Laporte G., McDonald E.G., Lee T.C. Non-invasive diagnosis of Pneumocystis jirovecii pneumonia: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2022;28:23–30. doi: 10.1016/j.cmi.2021.08.017. - DOI - PubMed
LinkOut - more resources
Full Text Sources

