The Plant Defensin Ppdef1 Is a Novel Topical Treatment for Onychomycosis
- PMID: 37998916
- PMCID: PMC10672221
- DOI: 10.3390/jof9111111
The Plant Defensin Ppdef1 Is a Novel Topical Treatment for Onychomycosis
Abstract
Onychomycosis, or fungal nail infection, causes not only pain and discomfort but can also have psychological and social consequences for the patient. Treatment of onychomycosis is complicated by the location of the infection under the nail plate, meaning that antifungal molecules must either penetrate the nail or be applied systemically. Currently, available treatments are limited by their poor nail penetration for topical products or their potential toxicity for systemic products. Plant defensins with potent antifungal activity have the potential to be safe and effective treatments for fungal infections in humans. The cystine-stabilized structure of plant defensins makes them stable to the extremes of pH and temperature as well as digestion by proteases. Here, we describe a novel plant defensin, Ppdef1, as a peptide for the treatment of fungal nail infections. Ppdef1 has potent, fungicidal activity against a range of human fungal pathogens, including Candida spp., Cryptococcus spp., dermatophytes, and non-dermatophytic moulds. In particular, Ppdef1 has excellent activity against dermatophytes that infect skin and nails, including the major etiological agent of onychomycosis Trichophyton rubrum. Ppdef1 also penetrates human nails rapidly and efficiently, making it an excellent candidate for a novel topical treatment of onychomycosis.
Keywords: NMR; Ppdef1; Trichophyton rubrum; antifungal; onychomycosis; plant defensin.
Conflict of interest statement
N.L.v.d.W., J.A.M., S.P., Y.M.G. and M.A.A. are Hexima Ltd. shareholders. N.L.v.d.W. and M.A.A. are inventors of the method of treatment of fungal infections EP3209319B1 & US9713632B2. Hexima Ltd. has granted permission to publish the results. We confirm that neither the manuscript nor any parts of its content are currently under consideration or published in another journal.
Figures
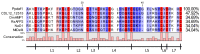
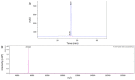
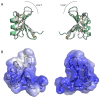
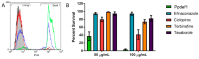




References
-
- Cañete-Gibas C., Wiederhold N.P. Mycology of Onychomycosis. Clin. Microbiol. Newsl. 2023;45:11–17. doi: 10.1016/j.clinmicnews.2023.01.002. - DOI
-
- Elewski B.E., Rich P., Pollak R., Pariser D.M., Watanabe S., Senda H., Ieda C., Smith K., Pillai R., Ramakrishna T., et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: Two phase III multicenter, randomized, double-blind studies. J. Am. Acad. Dermatol. 2013;68:600–608. doi: 10.1016/j.jaad.2012.10.013. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

