Advanced Hydrogel-Based Strategies for Enhanced Bone and Cartilage Regeneration: A Comprehensive Review
- PMID: 37998975
- PMCID: PMC10670584
- DOI: 10.3390/gels9110885
Advanced Hydrogel-Based Strategies for Enhanced Bone and Cartilage Regeneration: A Comprehensive Review
Abstract
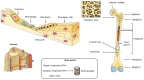
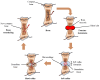
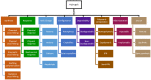
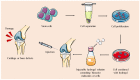
Bone and cartilage tissue play multiple roles in the organism, including kinematic support, protection of organs, and hematopoiesis. Bone and, above all, cartilaginous tissues present an inherently limited capacity for self-regeneration. The increasing prevalence of disorders affecting these crucial tissues, such as bone fractures, bone metastases, osteoporosis, or osteoarthritis, underscores the urgent imperative to investigate therapeutic strategies capable of effectively addressing the challenges associated with their degeneration and damage. In this context, the emerging field of tissue engineering and regenerative medicine (TERM) has made important contributions through the development of advanced hydrogels. These crosslinked three-dimensional networks can retain substantial amounts of water, thus mimicking the natural extracellular matrix (ECM). Hydrogels exhibit exceptional biocompatibility, customizable mechanical properties, and the ability to encapsulate bioactive molecules and cells. In addition, they can be meticulously tailored to the specific needs of each patient, providing a promising alternative to conventional surgical procedures and reducing the risk of subsequent adverse reactions. However, some issues need to be addressed, such as lack of mechanical strength, inconsistent properties, and low-cell viability. This review describes the structure and regeneration of bone and cartilage tissue. Then, we present an overview of hydrogels, including their classification, synthesis, and biomedical applications. Following this, we review the most relevant and recent advanced hydrogels in TERM for bone and cartilage tissue regeneration.
Keywords: advanced hydrogels; bone regeneration; extracellular matrix (ECM); scaffolds; stem cells (SCs); tissue engineering and regenerative medicine (TERM).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Wu A.-M., Bisignano C., James S.L., Abady G.G., Abedi A., Abu-Gharbieh E., Alhassan R.K., Alipour V., Arabloo J., Asaad M., et al. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990–2019: A systematic analysis from the Global Burden of Disease Study 2019. Lancet Health Longev. 2021;2:e580–e592. doi: 10.1016/S2666-7568(21)00172-0. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

