Characterization of the Bacterial Profile from Natural and Laboratory Glossina Populations
- PMID: 37999039
- PMCID: PMC10671886
- DOI: 10.3390/insects14110840
Characterization of the Bacterial Profile from Natural and Laboratory Glossina Populations
Abstract
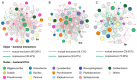
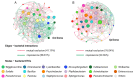
Tsetse flies (Glossina spp.; Diptera: Glossinidae) are viviparous flies that feed on blood and are found exclusively in sub-Saharan Africa. They are the only cyclic vectors of African trypanosomes, responsible for human African trypanosomiasis (HAT) and animal African trypanosomiasis (AAT). In this study, we employed high throughput sequencing of the 16S rRNA gene to unravel the diversity of symbiotic bacteria in five wild and three laboratory populations of tsetse species (Glossina pallidipes, G. morsitans, G. swynnertoni, and G. austeni). The aim was to assess the dynamics of bacterial diversity both within each laboratory and wild population in relation to the developmental stage, insect age, gender, and location. Our results indicated that the bacterial communities associated with the four studied Glossina species were significantly influenced by their region of origin, with wild samples being more diverse compared to the laboratory samples. We also observed that the larval microbiota was significantly different than the adults. Furthermore, the sex and the species did not significantly influence the formation of the bacterial profile of the laboratory colonies once these populations were kept under the same rearing conditions. In addition, Wigglesworthia, Acinetobacter, and Sodalis were the most abundant bacterial genera in all the samples, while Wolbachia was significantly abundant in G. morsitans compared to the other studied species. The operational taxonomic unit (OTU) co-occurrence network for each location (VVBD insectary, Doma, Makao, and Msubugwe) indicated a high variability between G. pallidipes and the other species in terms of the number of mutual exclusion and copresence interactions. In particular, some bacterial genera, like Wigglesworthia and Sodalis, with high relative abundance, were also characterized by a high degree of interactions.
Keywords: 16S rRNA; amplicon sequencing; bacterial profile; mass rearing; tsetse fly.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures










References
-
- Farrar J., Hotez P.J., Junghanss T., Lalloo D.G., White N.J., Manson P. In: Manson’s Tropical Diseases. 23rd ed. Kang G., editor. Elsevier/Saunders; Philadelphia, Pennsylvania: 2013.
-
- Dargie J. PAAT Tsetse and Trypanosomosis Information-INDEX-Volume 33, 34-Parts 1-2 (2010), 1-2 (2011)-Numbers 15196-16036. FAO; Rome, Italy: 2012.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

