Diffuse Optical Monitoring of Cerebral Hemodynamics and Oxygen Metabolism during and after Cardiopulmonary Bypass: Hematocrit Correction and Neurological Vulnerability
- PMID: 37999249
- PMCID: PMC10672802
- DOI: 10.3390/metabo13111153
Diffuse Optical Monitoring of Cerebral Hemodynamics and Oxygen Metabolism during and after Cardiopulmonary Bypass: Hematocrit Correction and Neurological Vulnerability
Abstract
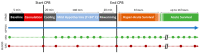
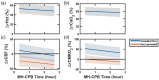
Cardiopulmonary bypass (CPB) provides cerebral oxygenation and blood flow (CBF) during neonatal congenital heart surgery, but the impacts of CPB on brain oxygen supply and metabolic demands are generally unknown. To elucidate this physiology, we used diffuse correlation spectroscopy and frequency-domain diffuse optical spectroscopy to continuously measure CBF, oxygen extraction fraction (OEF), and oxygen metabolism (CMRO2) in 27 neonatal swine before, during, and up to 24 h after CPB. Concurrently, we sampled cerebral microdialysis biomarkers of metabolic distress (lactate-pyruvate ratio) and injury (glycerol). We applied a novel theoretical approach to correct for hematocrit variation during optical quantification of CBF in vivo. Without correction, a mean (95% CI) +53% (42, 63) increase in hematocrit resulted in a physiologically improbable +58% (27, 90) increase in CMRO2 relative to baseline at CPB initiation; following correction, CMRO2 did not differ from baseline at this timepoint. After CPB initiation, OEF increased but CBF and CMRO2 decreased with CPB time; these temporal trends persisted for 0-8 h following CPB and coincided with a 48% (7, 90) elevation of glycerol. The temporal trends and glycerol elevation resolved by 8-24 h. The hematocrit correction improved quantification of cerebral physiologic trends that precede and coincide with neurological injury following CPB.
Keywords: cardiopulmonary bypass; cerebral hemodynamics; diffuse optics; hemoconcentration; mild hypothermia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources

