The Polyketides with Antimicrobial Activities from a Mangrove Endophytic Fungus Trichoderma lentiforme ML-P8-2
- PMID: 37999390
- PMCID: PMC10672575
- DOI: 10.3390/md21110566
The Polyketides with Antimicrobial Activities from a Mangrove Endophytic Fungus Trichoderma lentiforme ML-P8-2
Abstract
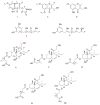
Five new polyketides, including two chromones (1-2), two phenyl derivatives (4-5), and a tandyukusin derivative (6), along with five known polyketides (3 and 7-10) were isolated from mangrove endophytic fungus Trichoderma lentiforme ML-P8-2. The planar structures of compounds were elucidated via detailed 1D, 2D NMR, and HR-ESI-MS analysis. ECD spectra, optical rotation values calculation, and alkali hydrolysis were applied in the determination of the absolute configuration of the new compounds. In bioassays, 6 and 9 exhibited promising antifungal activities against Penicillium italicum, with an MIC value of 6.25 μM for both compounds. Moreover, 3 displayed moderate AChE inhibitory activity with an IC50 value of 20.6 ± 0.3 μM.
Keywords: AChE inhibitory activity; Trichoderma lentiforme; antimicrobial activity; mangrove endophytic fungus; polyketide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


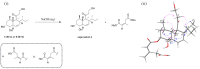
References
-
- Xu J. Bioactive natural products derived from mangrove-associated microbes. RSC Adv. 2015;5:841–892. doi: 10.1039/C4RA11756E. - DOI
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

