Noninvasive Vagus Nerve Stimulation in Postural Tachycardia Syndrome: A Randomized Clinical Trial
- PMID: 37999672
- PMCID: PMC10932945
- DOI: 10.1016/j.jacep.2023.10.015
Noninvasive Vagus Nerve Stimulation in Postural Tachycardia Syndrome: A Randomized Clinical Trial
Abstract
Background: Low-level transcutaneous stimulation of the auricular branch of the vagus nerve at the tragus is antiarrhythmic and anti-inflammatory in animals and humans. Preliminary studies show that transcutaneous vagus nerve stimulation (tVNS) is beneficial in animal models of postural tachycardia syndrome (POTS).
Objectives: In this study the authors conducted a sham-controlled, double-blind, randomized clinical trial to examine the effect of tVNS on POTS over a 2-month period relative to sham stimulation.
Methods: tVNS (20 Hz, 1 mA below discomfort threshold) was delivered using an ear clip attached to either the tragus (active; n = 12) or the ear lobe (sham; n = 14) for 1 hour daily over a 2-month period. Postural tachycardia was assessed during the baseline and 2-month visit. Heart rate variability based on 5-minute electrocardiogram, serum cytokines, and antiautonomic autoantibodies were measured at the respective time points.
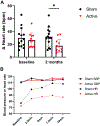
Results: Mean age was 34 ± 11 years (100% female; 81% Caucasian). Adherence to daily stimulation was 83% in the active arm and 86% in the sham arm (P > 0.05). Postural tachycardia was significantly less in the active arm compared with the sham arm at 2 months (mean postural increase in heart rate 17.6 ± 9.9 beats/min vs 31.7 ± 14.4 beats/min; P = 0.01). Antiadrenergic autoantibodies and inflammatory cytokines were lower in the active arm compared with the sham arm at 2 months (P < 0.05). Heart rate variability was better in the active arm. No device-related side effects were observed.
Conclusions: Our results support the emerging paradigm of noninvasive neuromodulation to treat POTS. Mechanistically, this effect appears to be related to reduction of antiautonomic autoantibodies and inflammatory cytokines, and improvement in autonomic tone. Further studies are warranted. (Autoimmune Basis for Postural Tachycardia Syndrome; NCT05043051).
Keywords: autoantibodies; autonomic modulation; inflammation; postural tachycardia syndrome.
Copyright © 2024 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding Support and Author Disclosures This work was supported by National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute R01HL161008 to Dr Stavrakis and R01HL128393 to Dr Yu, NIH/National Institute of General Medical Sciences 1U54GM10493, and individual donations from Francie Fitzgerald and family through the OU Foundation Fund. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures




Comment in
-
Emerging Role of Autonomic Modulation by Transcutaneous Vagus Nerve Stimulation: Electrifying Hope in POTS?JACC Clin Electrophysiol. 2024 Feb;10(2):356-358. doi: 10.1016/j.jacep.2023.11.024. Epub 2024 Jan 31. JACC Clin Electrophysiol. 2024. PMID: 38300209 No abstract available.
References
-
- Bryarly M, Phillips LT, Fu Q, Vernino S, Levine BD. Postural orthostatic tachycardia syndrome: JACC Focus Seminar. J Am Coll Cardiol. 2019;73:1207–1228. - PubMed
-
- Mar PL, Raj SR. Postural orthostatic tachycardia syndrome: mechanisms and new therapies. Annu Rev Med. 2020;71:235–248. - PubMed
-
- Grubb AF, Grubb BP. Postural orthostatic tachycardia syndrome: new concepts in pathophysiology and management. Trends Cardiovasc Med. 2023;33:65–69. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

