Long-Term Treatment Over 52 Weeks with Monthly Fremanezumab in Drug-Resistant Migraine: A Prospective Multicenter Cohort Study
- PMID: 37999868
- PMCID: PMC10703741
- DOI: 10.1007/s40263-023-01050-3
Long-Term Treatment Over 52 Weeks with Monthly Fremanezumab in Drug-Resistant Migraine: A Prospective Multicenter Cohort Study
Abstract
Background: Real-world studies on fremanezumab, an anti-calcitonin gene-related peptide monoclonal antibody for migraine prevention, are few and with limited follow-up.
Objective: We aimed to evaluate the long-term (up to 52 weeks) effectiveness and tolerability of fremanezumab in high-frequency episodic migraine and chronic migraine.
Methods: This s an independent, prospective, multicenter cohort study enrolling outpatients in 17 Italian Headache Centers with high-frequency episodic migraine or chronic migraine and multiple preventive treatment failures. Patients were treated with fremanezumab 225 mg monthly. The primary outcomes included changes from baseline (1 month before treatment) in monthly headache days, response rates (reduction in monthly headache days from baseline), and persistence in medication overuse at months 3, 6, and 12 (all outcome timeframes refer to the stated month). Secondary outcomes included changes from baseline in acute medication intake and disability questionnaires scores at the same timepoints. A last observation carried forward analysis was also performed.
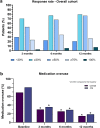
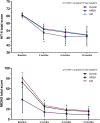
Results: A total of 90 patients who received at least one dose of fremanezumab and with a potential 12-month follow-up were included. Among them, 15 (18.0%) patients discontinued treatment for the entire population, a reduction in monthly headache days compared with baseline was reported at month 3, with a significant median [interquartile range] reduction in monthly headache days (- 9.0 [11.5], p < 0.001). A statistically different reduction was also reported at month 6 compared with baseline (- 10.0 [12.0]; p < 0.001) and at 12 months of treatment (- 10.0 [14.0]; p < 0.001). The percentage of patients with medication overuse was significantly reduced compared with baseline from 68.7% (57/83) to 29.6% (24/81), 25.3% (19/75), and 14.7% (10/68) at 3, 6, and 12 months of treatment, respectively (p < 0.001). Acute medication use (days and total number) and disability scores were also significantly reduced (p < 0.001). A ≥ 50% response rate was achieved for 51.9, 67.9, and 76.5% of all patients at 3, 6, and 12 months, respectively. Last observation carried forward analyses confirmed these findings. Fremanezumab was well tolerated, with just one patient discontinuing treatment because of adverse events.
Conclusions: This study provides evidence for the real-world effectiveness of fremanezumab in treating both high-frequency episodic migraine and chronic migraine, with meaningful and sustained improvements in multiple migraine-related variables. No new safety issue was identified.
© 2023. The Author(s).
Conflict of interest statement
Pierangelo Geppetti received personal fees from Allergan, Eli Lilly, Novartis, Amgen, and TEVA; grants from Amgen, TEVA, Eli-Lilly, Allergan, and Chiesi; is on the Scientific Advisory Board for Endosome Therapeutics; and is a founding scientist of FloNext srl, Spinoff of the University of Florence. Francesco De Cesaris received personal fees from TEVA, Eli Lilly, and Novartis. Luigi Francesco Iannone received personal fees from Eli-Lilly and TEVA, and a travel grant from Lundbeck. Simona Sacco reports personal fees as a speaker or advisor from Abbott, Allergan-AbbVie, AstraZeneca, Eli Lilly, Lundbeck, Novartis, NovoNordisk, Pfizer, and Teva, and research grants from Novartis. Raffaele Ornello reports personal fees from Novartis, Eli Lilly, and Teva, and non-financial support from Novartis, Allergan, Eli Lilly, and Teva. Simona Guerzoni received personal fees from Allergan/AbbVie, Eli Lilly, Novartis, Teva, and Lundbeck. Carlo Baraldi received fees and honoraria from Eli-Lilly, TEVA, Allergan/AbbVie, Lundbeck, and Novartis. Grazia Sances received personal fees as a speaker or was on the Advisory Board for Eli-Lilly, Novartis, TEVA, Lundbeck, and Pfizer. Roberto De Icco received honoraria for scientific presentations from Eli-Lilly and Teva. Gloria Vaghi received travel grants from Lundbeck and TEVA. Marta Allena received personal fees as a speaker from Eli Lilly. N.G. received personal fees as a speaker from Eli Lilly. Valeria Caponnetto, Antonio Russo, Marcello Silvestro, Alessandro Tessitore, Cristina Tassorelli, Grazia Sances, Flavia Lo Castro, Simona Guerzoni, Maria Pia Prudenzano, Adriana Fallacara, Martino Gentile, Agnese Onofri, Andrea Burgalassi, Alberto Chiarugi, Antonio Granato, Alfonsina Casalena, Marina De Tommaso, Edoardo Mampreso, Paola Merlo, Gianluca Coppola, Stefania Battistini, Valentina Rebecchi, Innocenzo Rainero, Federica Nicoletta Sepe, and Giorgio Dalla Volta have no conflicts of interest that are directly relevant to the content of this article.
Figures


References
-
- Ferrari MD, Diener HC, Ning X, Galic M, Cohen JM, Yang R, et al. Fremanezumab versus placebo for migraine prevention in patients with documented failure to up to four migraine preventive medication classes (FOCUS): a randomised, double-blind, placebo-controlled, phase 3b trial. Lancet. 2019;394(10203):1030–1040. doi: 10.1016/S0140-6736(19)31946-4. - DOI - PubMed
-
- Ashina M, Cohen JM, Galic M, Campos VR, Barash S, Ning X, et al. Efficacy and safety of fremanezumab in patients with episodic and chronic migraine with documented inadequate response to 2 to 4 classes of migraine preventive medications over 6 months of treatment in the phase 3b FOCUS study. J Headache Pain. 2021;22(1):68. doi: 10.1186/s10194-021-01279-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

