The Primary Prevention of Poststroke Epilepsy in Patients With Middle Cerebral Artery Infarct: Protocol for a Randomized Controlled Trial
- PMID: 37999939
- PMCID: PMC10709784
- DOI: 10.2196/49412
The Primary Prevention of Poststroke Epilepsy in Patients With Middle Cerebral Artery Infarct: Protocol for a Randomized Controlled Trial
Abstract
Background: Poststroke epilepsy poses a significant clinical challenge for individuals recovering from strokes, leading to a less favorable long-term outlook and increased mortality rates. Existing studies have primarily concentrated on administering antiseizure or anticonvulsant treatments only after the onset of late-onset seizures, without intervening during the epileptogenesis phase following a stroke.
Objective: This research protocol is designed to conduct a randomized controlled trial to assess whether the early, preventive introduction of low-dose antiepileptic drug therapy (levetiracetam [LEV] or perampanel [PER]) in patients who have experienced middle cerebral artery (MCA) infarction can reduce the risk of developing poststroke epilepsy (primary prevention).
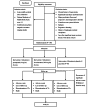
Methods: Participants with MCA infarction, either with or without reperfusion treatments, will be recruited and promptly receive preventive intervention within 72 hours of the stroke occurrence. These participants will be randomly assigned to receive either PER (4 mg per day), LEV (1000 mg per day), or a placebo that matches the active drugs. This treatment will continue for 12 weeks after allocation. Brain magnetic resonance imaging will be used to confirm the presence of MCA territory infarction, and an electroencephalography will be used to ensure the absence of epileptiform discharges or electrographic seizures at the time of the stroke. All participants will undergo follow-up assessments for 72 weeks after allocation.
Results: The primary outcome under evaluation will be the incidence of poststroke epilepsy in the 3 groups following the 18-month study period. Secondary outcomes will encompass the time to the occurrence of the first seizure, the severity of seizures, any treatment-related adverse events, and the modified Rankin scale score at 3 and 18 months. Exploratory outcomes will involve comparing the effectiveness and safety of PER and LEV.
Conclusions: We anticipate that the intervention groups will experience a lower incidence and reduced severity of poststroke epilepsy compared to the control group after 18 months. We aim to establish evidence supporting the potential preventive effects of LEV and PER on poststroke seizures and epilepsy in patients with MCA infarction, as well as to explore the antiepileptogenic potential of both LEV and PER in patients with major ischemic strokes.
Trial registration: ClinicalTrials.gov NCT04858841; https://clinicaltrials.gov/study/NCT04858841.
International registered report identifier (irrid): DERR1-10.2196/49412.
Keywords: angina; cardiologist; cardiology; cardiovascular; development; drug therapy; efficacy; epilepsy; heart attack; middle cerebral artery infarct; mortality; poststroke epilepsy; prognosis; randomized control trial; seizure; seizure severity; stroke; stroke survivor.
©Yu-Shiue Chen, Pi-Shan Sung, Ming-Chi Lai, Chin-Wei Huang. Originally published in JMIR Research Protocols (https://www.researchprotocols.org), 24.11.2023.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures

Similar articles
-
Study protocol for a phase II randomised, double-blind, placebo-controlled trial of perampanel as an antiepileptogenic treatment following acute stroke.BMJ Open. 2021 May 10;11(5):e043488. doi: 10.1136/bmjopen-2020-043488. BMJ Open. 2021. PMID: 33972334 Free PMC article.
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Transient and permanent arterial occlusions modeling poststroke epilepsy in aging rats.Epilepsy Res. 2018 Dec;148:69-77. doi: 10.1016/j.eplepsyres.2018.10.012. Epub 2018 Oct 26. Epilepsy Res. 2018. PMID: 30391633
-
Topiramate versus carbamazepine monotherapy for epilepsy: an individual participant data review.Cochrane Database Syst Rev. 2019 Jun 24;6(6):CD012065. doi: 10.1002/14651858.CD012065.pub3. Cochrane Database Syst Rev. 2019. PMID: 31233229 Free PMC article.
-
Transcranial magnetic stimulation for the treatment of epilepsy.Cochrane Database Syst Rev. 2021 Apr 15;4(4):CD011025. doi: 10.1002/14651858.CD011025.pub3. Cochrane Database Syst Rev. 2021. PMID: 33884611 Free PMC article.
References
-
- Shetty AK. Prospects of levetiracetam as a neuroprotective drug against status epilepticus, traumatic brain injury, and stroke. Front Neurol. 2013;4:172. doi: 10.3389/fneur.2013.00172. https://europepmc.org/abstract/MED/24204362 - DOI - PMC - PubMed
-
- Zelano J, Holtkamp M, Agarwal N, Lattanzi S, Trinka E, Brigo F. How to diagnose and treat post-stroke seizures and epilepsy. Epileptic Disord. 2020;22(3):252–263. doi: 10.1684/epd.2020.1159. https://onlinelibrary.wiley.com/doi/10.1684/epd.2020.1159 epd.2020.1159 - DOI - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

