CDK4/6 inhibition enhances SHP2 inhibitor efficacy and is dependent upon RB function in malignant peripheral nerve sheath tumors
- PMID: 38000020
- PMCID: PMC10672174
- DOI: 10.1126/sciadv.adg8876
CDK4/6 inhibition enhances SHP2 inhibitor efficacy and is dependent upon RB function in malignant peripheral nerve sheath tumors
Abstract
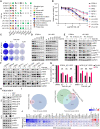
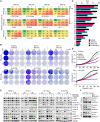
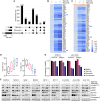
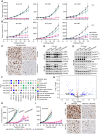
Malignant peripheral nerve sheath tumors (MPNSTs) are highly aggressive soft tissue sarcomas with limited treatment options, and new effective therapeutic strategies are desperately needed. We observe antiproliferative potency of genetic depletion of PTPN11 or pharmacological inhibition using the SHP2 inhibitor (SHP2i) TNO155. Our studies into the signaling response to SHP2i reveal that resistance to TNO155 is partially mediated by reduced RB function, and we therefore test the addition of a CDK4/6 inhibitor (CDK4/6i) to enhance RB activity and improve TNO155 efficacy. In combination, TNO155 attenuates the adaptive response to CDK4/6i, potentiates its antiproliferative effects, and converges on enhancement of RB activity, with greater suppression of cell cycle and inhibitor-of-apoptosis proteins, leading to deeper and more durable antitumor activity in in vitro and in vivo patient-derived models of MPNST, relative to either single agent. Overall, our study provides timely evidence to support the clinical advancement of this combination strategy in patients with MPNST and other tumors driven by loss of NF1.
Figures



Update of
-
CDK4/6 inhibition enhances SHP2 inhibitor efficacy and is dependent upon restoration of RB function in malignant peripheral nerve sheath tumors.bioRxiv [Preprint]. 2023 Feb 3:2023.02.02.526674. doi: 10.1101/2023.02.02.526674. bioRxiv. 2023. Update in: Sci Adv. 2023 Nov 24;9(47):eadg8876. doi: 10.1126/sciadv.adg8876. PMID: 36778419 Free PMC article. Updated. Preprint.
References
-
- B. S. Ducatman, B. W. Scheithauer, D. G. Piepgras, H. M. Reiman, D. M. Ilstrup, Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer 57, 2006–2021 (1986). - PubMed
-
- W. Lee, S. Teckie, T. Wiesner, L. Ran, C. N. Prieto Granada, M. Lin, S. Zhu, Z. Cao, Y. Liang, A. Sboner, W. D. Tap, J. A. Fletcher, K. H. Huberman, L. X. Qin, A. Viale, S. Singer, D. Zheng, M. F. Berger, Y. Chen, C. R. Antonescu, P. Chi, PRC2 is recurrently inactivated through EED or SUZ12 loss in malignant peripheral nerve sheath tumors. Nat. Genet. 46, 1227–1232 (2014). - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

