Molecular mechanisms underlying NLRP3 inflammasome activation and IL-1β production in air pollution fine particulate matter (PM2.5)-primed macrophages
- PMID: 38000727
- PMCID: PMC10804998
- DOI: 10.1016/j.envpol.2023.122997
Molecular mechanisms underlying NLRP3 inflammasome activation and IL-1β production in air pollution fine particulate matter (PM2.5)-primed macrophages
Abstract
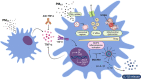
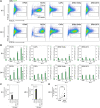
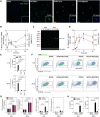
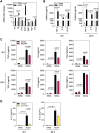
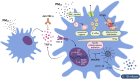
Exposure to air pollution fine particulate matter (PM2.5) aggravates respiratory and cardiovascular diseases. It has been proposed that PM2.5 uptake by alveolar macrophages promotes local inflammation that ignites a systemic response, but precise underlying mechanisms remain unclear. Here, we demonstrate that PM2.5 phagocytosis leads to NLRP3 inflammasome activation and subsequent release of the pro-inflammatory master cytokine IL-1β. Inflammasome priming and assembly was time- and dose-dependent in inflammasome-reporter THP-1-ASC-GFP cells, and consistent across PM2.5 samples of variable chemical composition. While inflammasome activation was promoted by different PM2.5 surrogates, significant IL-1β release could only be observed after stimulation with transition-metal rich Residual Oil Fly Ash (ROFA) particles. This effect was confirmed in primary human monocyte-derived macrophages and murine bone marrow-derived macrophages (BMDMs), and by confocal imaging of inflammasome-reporter ASC-Citrine BMDMs. IL-1β release by ROFA was dependent on the NLRP3 inflammasome, as indicated by lack of IL-1β production in ROFA-exposed NLRP3-deficient (Nlrp3-/-) BMDMs, and by specific NLRP3 inhibition with the pharmacological compound MCC950. In addition, while ROFA promoted the upregulation of pro-inflammatory gene expression and cytokines release, MCC950 reduced TNF-α, IL-6, and CCL2 production. Furthermore, inhibition of TNF-α with a neutralizing antibody decreased IL-1β release in ROFA-exposed BMDMs. Using electron tomography, ROFA particles were observed inside intracellular vesicles and mitochondria, which showed signs of ultrastructural damage. Mechanistically, we identified lysosomal rupture, K+ efflux, and impaired mitochondrial function as important prerequisites for ROFA-mediated IL-1β release. Interestingly, specific inhibition of superoxide anion production (O2•-) from mitochondrial respiratory Complex I, but not III, blunted IL-1β release in ROFA-exposed BMDMs. Our findings unravel the mechanism by which PM2.5 promotes IL-1β release in macrophages and provide a novel link between innate immune response and exposure to air pollution PM2.5.
Keywords: Inflammation; K(+) efflux; Lysosomal disruption; Macrophages; Mitochondria; Particulate matter.
Copyright © 2023 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Afonina I.S., Zhong Z., Karin M., Beyaert R. Limiting inflammation-the negative regulation of NF-kappaB and the NLRP3 inflammasome. Nat. Immunol. 2017;18:861–869. - PubMed
-
- Brand M.D. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic. Biol. Med. 2016;100:14–31. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

