Long-term performance of single-connector (DF4) implantable defibrillator leads
- PMID: 38000900
- PMCID: PMC10751803
- DOI: 10.1093/europace/euad347
Long-term performance of single-connector (DF4) implantable defibrillator leads
Abstract
Aims: Single-connector (DF4) defibrillator leads have become the predominantly implanted transvenous implantable cardioverter-defibrillator lead. However, data on their long-term performance are derived predominantly from manufacturer product performance reports.
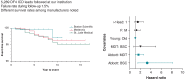
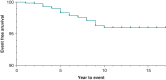
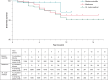
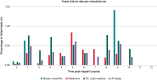
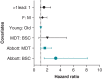
Methods and results: We reviewed medical records in 5289 patients with DF4 leads between 2011 and 2023 to determine the frequency of lead-related abnormalities. We defined malfunction as any single or combination of electrical abnormalities requiring revision including a sudden increase (≥2×) in stimulation threshold, a discrete jump in high-voltage impedance, or sensing of non-physiologic intervals or noise. We documented time to failure, predictors of failure, and management strategies. Mean follow-up after implant was 4.15 ± 3.6 years (median = 3.63), with 37% of leads followed for >5 years. A total of 80 (1.5%) leads demonstrated electrical abnormalities requiring revision with an average time to failure of 4 ± 2.8 years (median = 3.5). Of the leads that malfunctioned, 62/80 (78%) were extracted and replaced with a new lead and in the other 18 cases, malfunctioned DF4 leads were abandoned, and a new lead implanted. In multivariable models, younger age at implant (OR 1.03 per year; P < 0.001) and the presence of Abbott/St. Jude leads increased the risk of malfunction.
Conclusion: DF4 defibrillator leads demonstrate excellent longevity with >98.3% of leads followed for at least 5 years still functioning normally. Younger age at implant and lead manufacturer are associated with an increased risk of DF4 lead malfunction. The differences in lead survival between manufacturers require further investigation.
Keywords: DF1 leads; DF4 leads; ICD; Lead failure; Lead revision.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: A.d.S.: honoraria/speaking/consulting fee—Baylis Medical Company. M.S.L.: honoraria/speaking/consulting fee—Medtronic; Boston Scientific. D.B.D.L.: honoraria/speaking/consulting fee—Boston Scientific; Medtronic; AtriCure, Inc. A.M.P.: honoraria/speaking/consulting fee—Biosense Webster, Inc. M.F.E.-C.: honoraria/speaking/consulting fee—Boston Scientific; Medtronic. The rest of the authors have no disclosures
Figures
References
-
- Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877–83. - PubMed
-
- Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005;352:225–37. - PubMed
-
- Hauser RG, Maron BJ. Lessons from the failure and recall of an implantable cardioverter-defibrillator. Circulation 2005;112:2040–2. - PubMed
-
- Gornick CC, Hauser RG, Almquist AK, Maron BJ. Unpredictable implantable cardioverter-defibrillator pulse generator failure due to electrical overstress causing sudden death in a young high-risk patient with hypertrophic cardiomyopathy. Heart Rhythm 2005;2:681–3. - PubMed
-
- Brinker JA. Implantable cardioverter-defibrillator lead failure: how weak is the link? Nat Clin Pract Cardiovasc Med 2008;5:758–9. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical