Finishing the egg
- PMID: 38000906
- PMCID: PMC10763546
- DOI: 10.1093/genetics/iyad183
Finishing the egg
Abstract
Gamete development is a fundamental process that is highly conserved from early eukaryotes to mammals. As germ cells develop, they must coordinate a dynamic series of cellular processes that support growth, cell specification, patterning, the loading of maternal factors (RNAs, proteins, and nutrients), differentiation of structures to enable fertilization and ensure embryonic survival, and other processes that make a functional oocyte. To achieve these goals, germ cells integrate a complex milieu of environmental and developmental signals to produce fertilizable eggs. Over the past 50 years, Drosophila oogenesis has risen to the forefront as a system to interrogate the sophisticated mechanisms that drive oocyte development. Studies in Drosophila have defined mechanisms in germ cells that control meiosis, protect genome integrity, facilitate mRNA trafficking, and support the maternal loading of nutrients. Work in this system has provided key insights into the mechanisms that establish egg chamber polarity and patterning as well as the mechanisms that drive ovulation and egg activation. Using the power of Drosophila genetics, the field has begun to define the molecular mechanisms that coordinate environmental stresses and nutrient availability with oocyte development. Importantly, the majority of these reproductive mechanisms are highly conserved throughout evolution, and many play critical roles in the development of somatic tissues as well. In this chapter, we summarize the recent progress in several key areas that impact egg chamber development and ovulation. First, we discuss the mechanisms that drive nutrient storage and trafficking during oocyte maturation and vitellogenesis. Second, we examine the processes that regulate follicle cell patterning and how that patterning impacts the construction of the egg shell and the establishment of embryonic polarity. Finally, we examine regulatory factors that control ovulation, egg activation, and successful fertilization.
Keywords: FlyBook; chorion gene amplification; egg activation; egg shape; eggshell; female reproductive tract; follicle cell differentiation; nutrient storage; oocyte maturation; ovulation.
© The Author(s) 2023. Published by Oxford University Press on behalf of The Genetics Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Conflicts of interest statement The author(s) declare no conflict of interest.
Figures

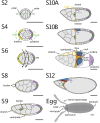
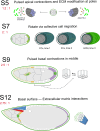
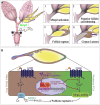
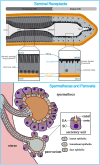
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

